Aqueous sulfuric acid (H, SO,) reacts with solid sodium hydroxide (NaOH) to produce aqueous sodium sulfate (Na, SO4) and liquid water (H,0). If 2.36 g of water is produced from the reaction of 19.6 g of sulfuric acid and 6.88 g of sodium hydroxide, calculate the percent yield of water. Round your answer to 3 significant figures.
Aqueous sulfuric acid (H, SO,) reacts with solid sodium hydroxide (NaOH) to produce aqueous sodium sulfate (Na, SO4) and liquid water (H,0). If 2.36 g of water is produced from the reaction of 19.6 g of sulfuric acid and 6.88 g of sodium hydroxide, calculate the percent yield of water. Round your answer to 3 significant figures.
Chapter3: Stoichiometry
Section: Chapter Questions
Problem 159CWP: Sulfur dioxide gas reacts with sodium hydroxide to form sodium sulfite and water. The unbalanced...
Related questions
Question
100%
please use the photo attached for the information
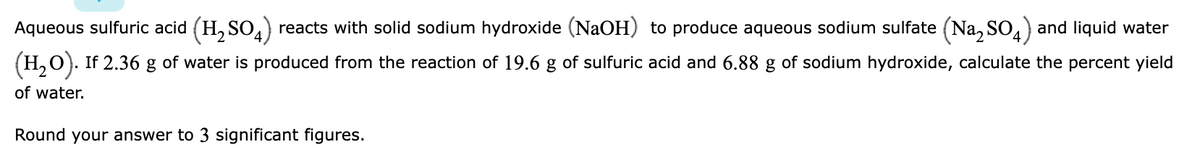
Transcribed Image Text:Aqueous sulfuric acid (H, SO,) reacts with solid sodium hydroxide (NaOH) to produce aqueous sodium sulfate (Na, SO,) and liquid water
(H,O). If 2.36
of water is produced from the reaction of 19.6 g of sulfuric acid and 6.88 g of sodium hydroxide, calculate the percent yield
of water.
Round your answer to 3 significant figures.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning