assignmentProblemID=142966924&offset3=next CHE154-H Gen Chem II Bronikowski S20 Part A Determine the percent ionization of a 0.245 M solution of benzoic acid (K, = 6.5 x 1) Express your answer using two significant figures. > View Available Hint(s) 1 ΑΣφ ха Хь х IXI X•10" Submit Previous Answers
assignmentProblemID=142966924&offset3=next CHE154-H Gen Chem II Bronikowski S20 Part A Determine the percent ionization of a 0.245 M solution of benzoic acid (K, = 6.5 x 1) Express your answer using two significant figures. > View Available Hint(s) 1 ΑΣφ ха Хь х IXI X•10" Submit Previous Answers
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.10QAP
Related questions
Question
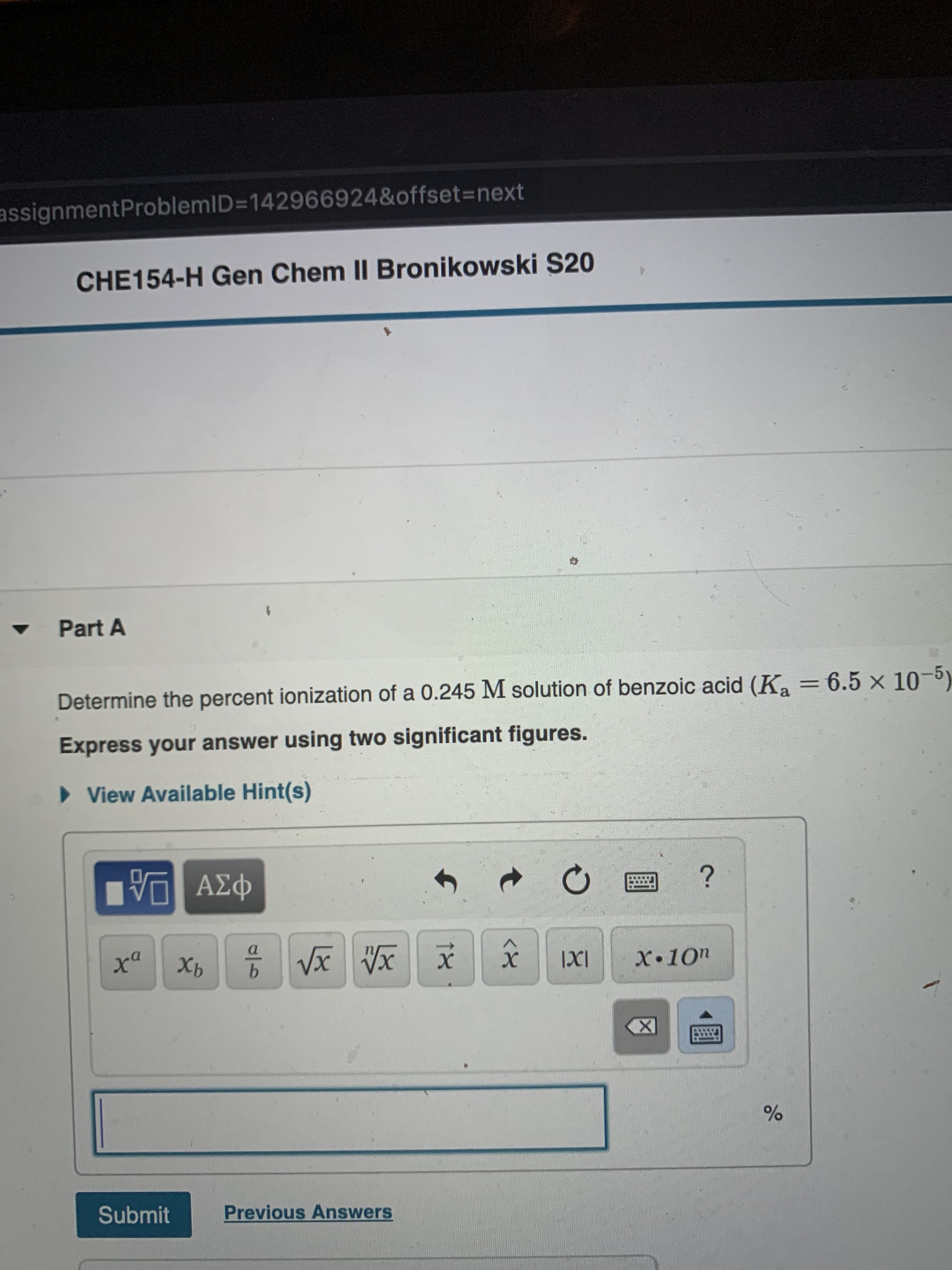
Transcribed Image Text:assignmentProblemID=142966924&offset3=next
CHE154-H Gen Chem II Bronikowski S20
Part A
Determine the percent ionization of a 0.245 M solution of benzoic acid (K, = 6.5 x 1)
Express your answer using two significant figures.
> View Available Hint(s)
1 ΑΣφ
ха
Хь
х
IXI
X•10"
Submit
Previous Answers
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Recommended textbooks for you



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning