Assume that you have an “X" solution that you do not know its concentration. But you have other X solutions with known concentrations and you know X solution absorbs maximum light at 575 nm. To calculate the unknown solution concentration, you have done some spectrophotometric obtained data given below. measurements and
Assume that you have an “X" solution that you do not know its concentration. But you have other X solutions with known concentrations and you know X solution absorbs maximum light at 575 nm. To calculate the unknown solution concentration, you have done some spectrophotometric obtained data given below. measurements and
Anatomy & Physiology
1st Edition
ISBN:9781938168130
Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Chapter3: The Cellular Level Of Organization
Section: Chapter Questions
Problem 7RQ: The diffusion of substances within a solution tends to move those substances ________ their ________...
Related questions
Question
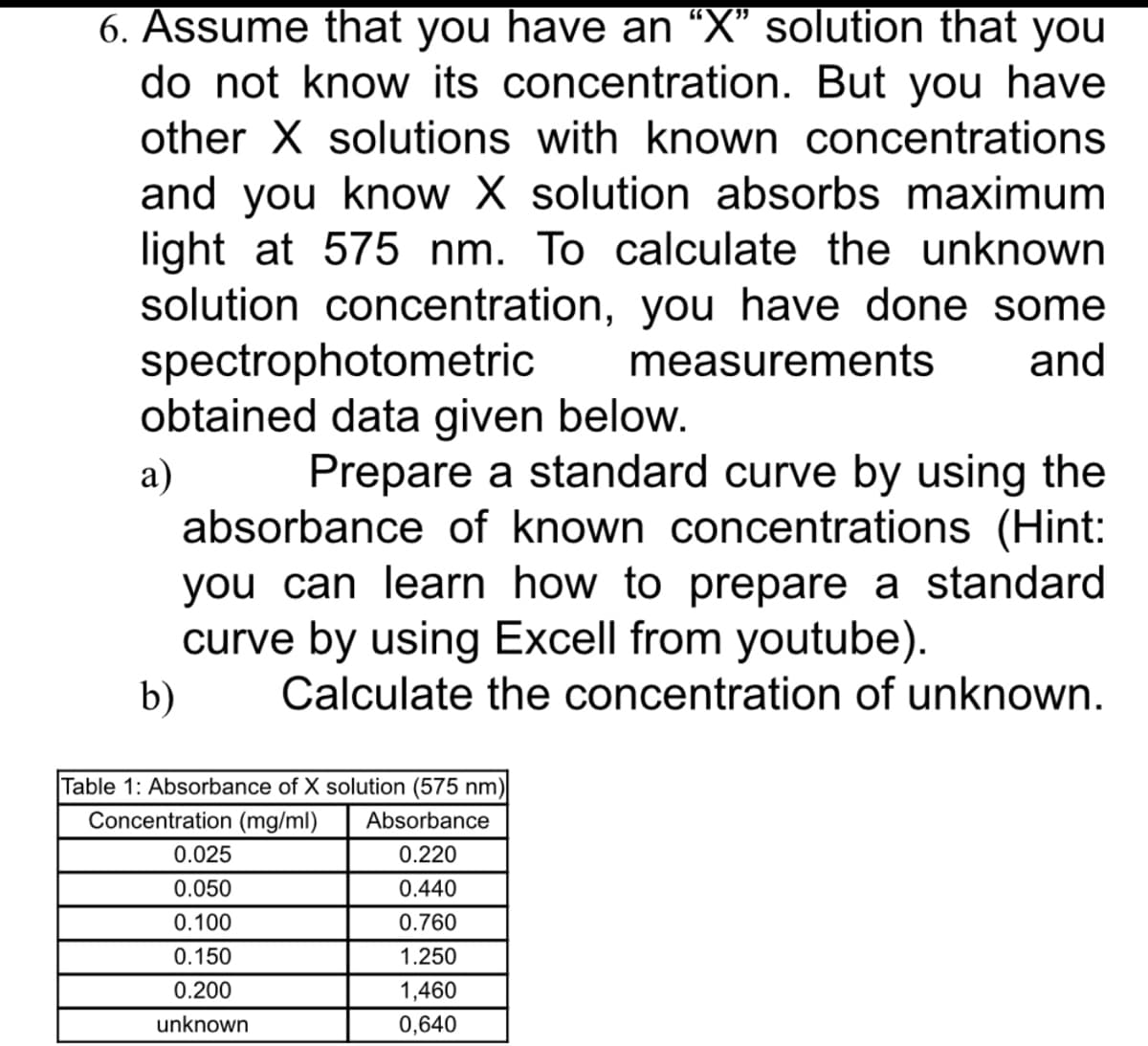
Transcribed Image Text:6. Assume that you have an “X" solution that you
do not know its concentration. But you have
other X solutions with known concentrations
and you know X solution absorbs maximum
light at 575 nm. To calculate the unknown
solution concentration, you have done some
spectrophotometric
obtained data given below.
measurements
and
Prepare a standard curve by using the
a)
absorbance of known concentrations (Hint:
you can learn how to prepare a standard
curve by using Excell from youtube).
Calculate the concentration of unknown.
b)
Table 1: Absorbance of X solution (575 nm)
Concentration (mg/ml)
Absorbance
0.025
0.220
0.050
0.440
0.100
0.760
0.150
1.250
0.200
1,460
unknown
0,640
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Anatomy & Physiology
Biology
ISBN:
9781938168130
Author:
Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:
OpenStax College

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax

Anatomy & Physiology
Biology
ISBN:
9781938168130
Author:
Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:
OpenStax College

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax