At 298 K, the equilibrium constant for the reaction Fe,O3(s) + 3 H2(g) 2 2 Fe(s) + 3 H¿O(€) is 4.0 × 10-6, and that for CO2(g) + H2(g) 2 CO(g) + H,O(€) is 3.2 x 10-4. Suppose some solid Fe,O3, solid Fe, and liquid H2O are brought into equilibrium with CO(g) and CO2(g) in a closed container at 298 K. Calculate the ratio of the partial pressure of CO(g) to that of CO2(g) at equilibrium.
At 298 K, the equilibrium constant for the reaction Fe,O3(s) + 3 H2(g) 2 2 Fe(s) + 3 H¿O(€) is 4.0 × 10-6, and that for CO2(g) + H2(g) 2 CO(g) + H,O(€) is 3.2 x 10-4. Suppose some solid Fe,O3, solid Fe, and liquid H2O are brought into equilibrium with CO(g) and CO2(g) in a closed container at 298 K. Calculate the ratio of the partial pressure of CO(g) to that of CO2(g) at equilibrium.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter12: Gaseous Chemical Equilibrium
Section: Chapter Questions
Problem 36QAP: At a certain temperature, K=0.29 for the decomposition of two moles of iodine trichloride, ICl3(s),...
Related questions
Question
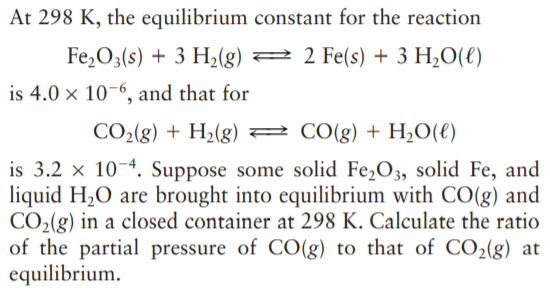
Transcribed Image Text:At 298 K, the equilibrium constant for the reaction
Fe,O3(s) + 3 H2(g) 2 2 Fe(s) + 3 H¿O(€)
is 4.0 × 10-6, and that for
CO2(g) + H2(g) 2 CO(g) + H,O(€)
is 3.2 x 10-4. Suppose some solid Fe,O3, solid Fe, and
liquid H2O are brought into equilibrium with CO(g) and
CO2(g) in a closed container at 298 K. Calculate the ratio
of the partial pressure of CO(g) to that of CO2(g) at
equilibrium.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning