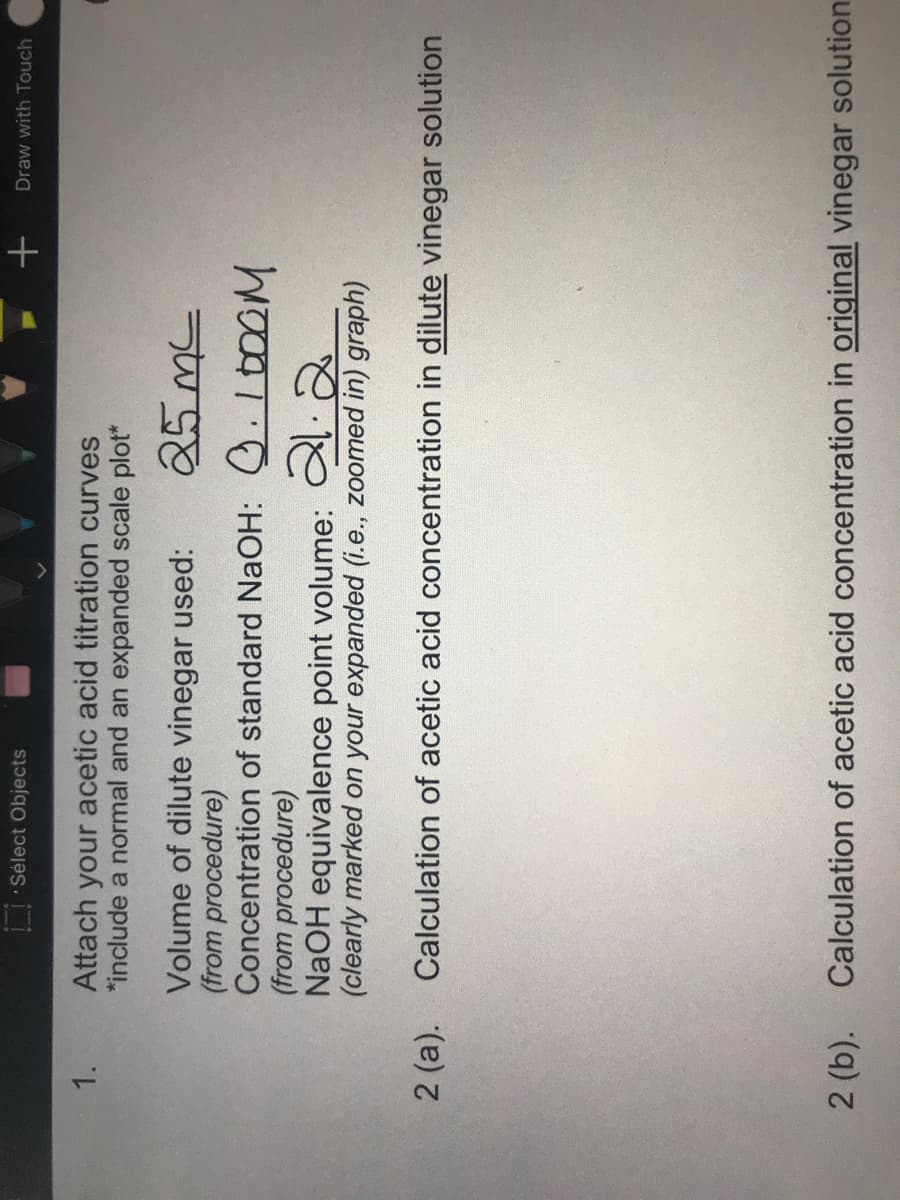
Attach your acetic acid titration curves *include a normal and an expanded scale plot* 1. Volume of dilute vinegar used: (from procedure) Concentration of standard NaOH: .1t00M (from procedure) NaOH equivalence point volume: 2 (clearly marked on your expanded (i.e., zoomed in) graph) 2 (a). Calculation of acetic acid concentration in dilute vinegar solution 2 (b). Calculation of acetic acid concentration in original vinegar solution
Attach your acetic acid titration curves *include a normal and an expanded scale plot* 1. Volume of dilute vinegar used: (from procedure) Concentration of standard NaOH: .1t00M (from procedure) NaOH equivalence point volume: 2 (clearly marked on your expanded (i.e., zoomed in) graph) 2 (a). Calculation of acetic acid concentration in dilute vinegar solution 2 (b). Calculation of acetic acid concentration in original vinegar solution
Chapter8: Polyfunctional Acids And Bases
Section: Chapter Questions
Problem 10P
Related questions
Question
100%
To find 2 (a) and (b): Are you supposed to use the volume of the dilute vinegar to find the conc. of the dilute vinegar? Is the moles of CH3COOH and NaOH, 1:1?
Transcribed Image Text:11Sélect Objects
Draw with Touch
Attach your acetic acid titration curves
*include a normal and an expanded scale plot*
1.
Volume of dilute vinegar used:
(from procedure)
Concentration of standard NaOH: .100OM
(from procedure)
NaOH equivalence point volume:
(clearly marked on your expanded (i.e., zoomed in) graph)
2 (a).
Calculation of acetic acid concentration in dilute vinegar solution
2 (b). Calculation of acetic acid concentration in original vinegar solution
![Determination of the [CH3COOH] in vinegar by titration a
dilute vinegar solution against a 0.1000 M NaOH solution
From the balanced equation:
CH,COOH + NaOH → H20 + Na*CH,CO-
& at the Equivalence Point:
moles CH,COOH = moles NAOH
%3D
Calculate the [CH;COOH]](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F9013faab-e249-4b7d-9250-32c104e024a0%2F3cdfb524-c622-4f9c-a76d-7fb2e626d09b%2Fpoa6eo4_processed.jpeg&w=3840&q=75)
Transcribed Image Text:Determination of the [CH3COOH] in vinegar by titration a
dilute vinegar solution against a 0.1000 M NaOH solution
From the balanced equation:
CH,COOH + NaOH → H20 + Na*CH,CO-
& at the Equivalence Point:
moles CH,COOH = moles NAOH
%3D
Calculate the [CH;COOH]
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



