b S2 ab S2 Part A Distinguish between the equilibrium constant expression and Kap for the dissolution of a sparingly soluble salt. The solubility-product constant does not explicitly contain the concentration of the insoluble salt, whereas the expression for the equilibrium constant has this concentration in the numerator. The solubility-product constant does not explicitly contain the concentration of the insoluble salt, whereas the expression for the equilibrium constant has this concentration in the denominator. The equilibrium constant does not explicitly contain the concentration of the insoluble salt, whereas the expression for the solubility-product constant has this concentration in the numerator. The equilibrium constant does not explicitly contain the concentration of the insoluble salt, whereas the expression for the solubility-product constant has this concentration in the denominator. Submit Request Answer Provide Feedback P Type here to search ESC F8 * TAB session.masteringchemistry.com/myct/itemView?assignmentProblemlD=D139247443 <07 Post-Lab S2 7.4 Post-Lab S2 Part A What are the greatest sources of error in this experiment? Select all that apply. V pH of water V Absorption of CO2 as solutions stand V Judgment of colors V Adding the incorrect amounts of liquid ORounding errors Submit Previous Answers Request Answer X Incorrect; Try Again; 4 attempts remaining Part B How could you minimize these sources of error? Select all that apply. Keep solutions covered Measure liquids with a large flask Use a pH meter to determine the pH of solutions Have a partner help judge color as well Submit Request Answer < Return to Assignment Provide Feedback P Type here to search
b S2 ab S2 Part A Distinguish between the equilibrium constant expression and Kap for the dissolution of a sparingly soluble salt. The solubility-product constant does not explicitly contain the concentration of the insoluble salt, whereas the expression for the equilibrium constant has this concentration in the numerator. The solubility-product constant does not explicitly contain the concentration of the insoluble salt, whereas the expression for the equilibrium constant has this concentration in the denominator. The equilibrium constant does not explicitly contain the concentration of the insoluble salt, whereas the expression for the solubility-product constant has this concentration in the numerator. The equilibrium constant does not explicitly contain the concentration of the insoluble salt, whereas the expression for the solubility-product constant has this concentration in the denominator. Submit Request Answer Provide Feedback P Type here to search ESC F8 * TAB session.masteringchemistry.com/myct/itemView?assignmentProblemlD=D139247443 <07 Post-Lab S2 7.4 Post-Lab S2 Part A What are the greatest sources of error in this experiment? Select all that apply. V pH of water V Absorption of CO2 as solutions stand V Judgment of colors V Adding the incorrect amounts of liquid ORounding errors Submit Previous Answers Request Answer X Incorrect; Try Again; 4 attempts remaining Part B How could you minimize these sources of error? Select all that apply. Keep solutions covered Measure liquids with a large flask Use a pH meter to determine the pH of solutions Have a partner help judge color as well Submit Request Answer < Return to Assignment Provide Feedback P Type here to search
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter17: Principles Of Chemical Reactivity: Other Aspects Of Aqueous Equilibria
Section17.6: Equilibria Involving Complex Ions
Problem 1.4ACP: Silver undergoes similar reactions as those shown for gold. Both metals react with cyanide ion in...
Related questions
Question
100%
Transcribed Image Text:b S2
ab S2
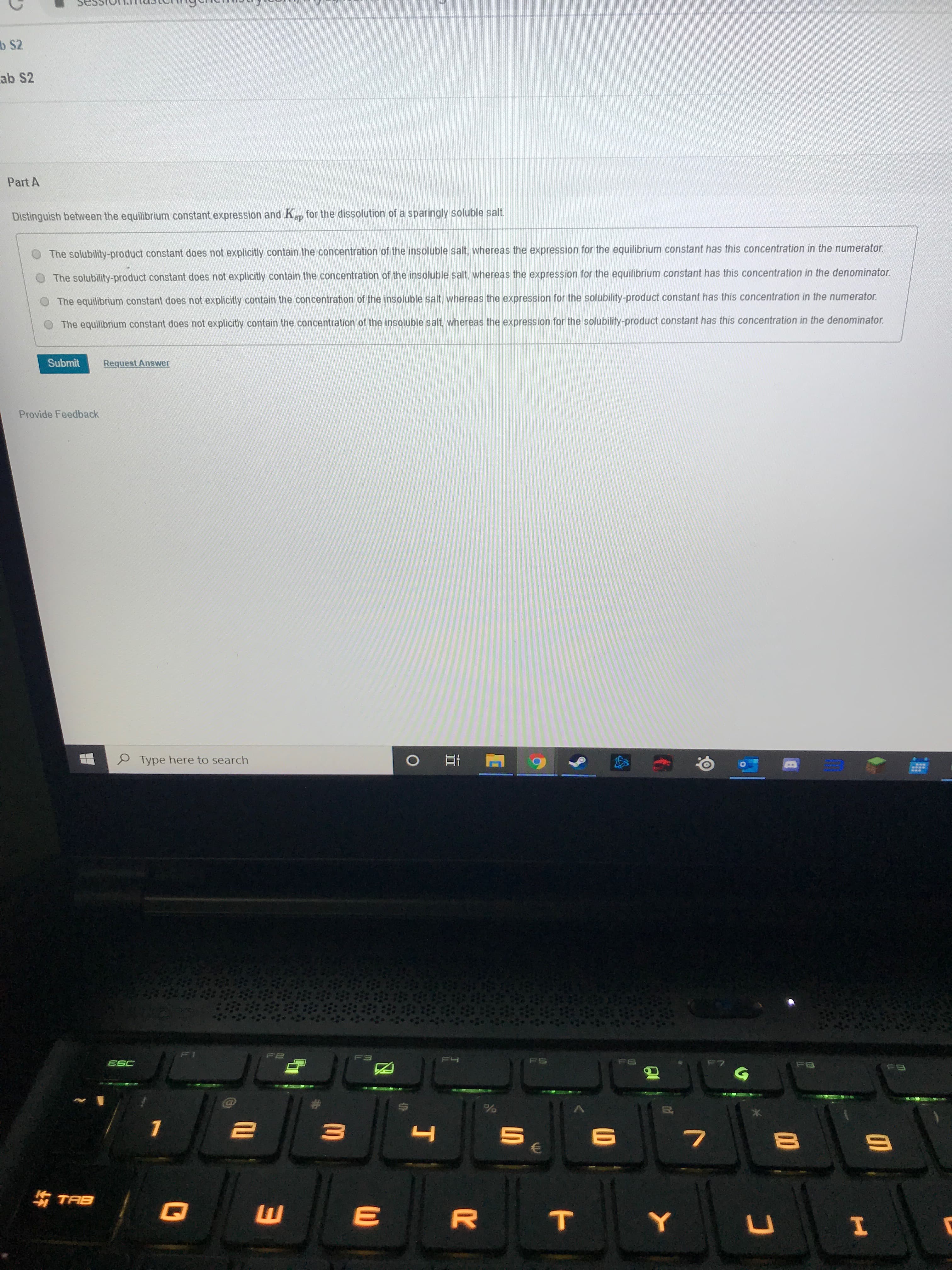
Part A
Distinguish between the equilibrium constant expression and Kap for the dissolution of a sparingly soluble salt.
The solubility-product constant does not explicitly contain the concentration of the insoluble salt, whereas the expression for the equilibrium constant has this concentration in the numerator.
The solubility-product constant does not explicitly contain the concentration of the insoluble salt, whereas the expression for the equilibrium constant has this concentration in the denominator.
The equilibrium constant does not explicitly contain the concentration of the insoluble salt, whereas the expression for the solubility-product constant has this concentration in the numerator.
The equilibrium constant does not explicitly contain the concentration of the insoluble salt, whereas the expression for the solubility-product constant has this concentration in the denominator.
Submit
Request Answer
Provide Feedback
P Type here to search
ESC
F8
* TAB
Transcribed Image Text:session.masteringchemistry.com/myct/itemView?assignmentProblemlD=D139247443
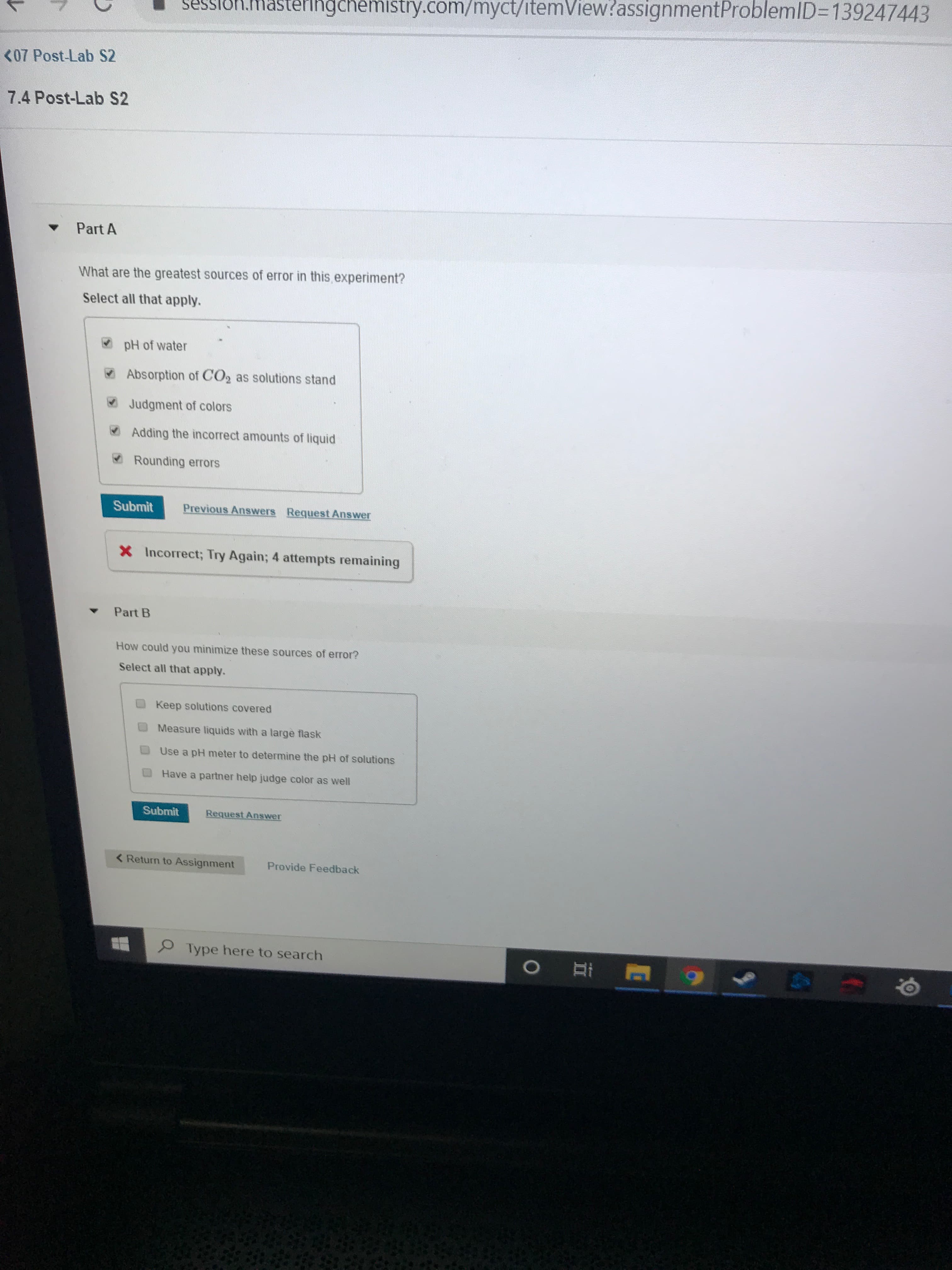
<07 Post-Lab S2
7.4 Post-Lab S2
Part A
What are the greatest sources of error in this experiment?
Select all that apply.
V pH of water
V Absorption of CO2 as solutions stand
V Judgment of colors
V Adding the incorrect amounts of liquid
ORounding errors
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 4 attempts remaining
Part B
How could you minimize these sources of error?
Select all that apply.
Keep solutions covered
Measure liquids with a large flask
Use a pH meter to determine the pH of solutions
Have a partner help judge color as well
Submit
Request Answer
< Return to Assignment
Provide Feedback
P Type here to search
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax
