(b) To the right is a diagram of the chemical groups in carboxymethyl-cellulose and DEAE-cellulose that are used for ion exchange chromatography to purify proteins. At pH 8 which chromatographic resin has to be used to separate iso-1-cytochrome c from iso-2- cytochrome c from a lysate of yeast cells? If the proteins are separated by application of a concentration gradient carboxymethyl of NaCl applied to the column, which ion Na® or Cle dis- places the proteins from the binding sites on the chroma- tographic resin? cellulose OH H3C CH3 ΘΝΗ HO HỌC, NH H3C 2-(diethylamino)ethyl cellulose (DEAE cellulose) Which protein iso-1-cytochrome c or iso-2-cytochrome c will be the first to be eluted from the column and why? Both cytochromes in their ferrous form are substrates of yeast cytochrome c peroxidase (CCP) and react optimally with this enzyme at pH 8 forming a Michaelis complex of 1:1 stoichiometry (CcP:cyt c). If the isoionic point of the peroxidase is 5.25, what must be the overall electrostatic charge on the peroxidase enzyme for this reaction to occur, positive or negative?
(b) To the right is a diagram of the chemical groups in carboxymethyl-cellulose and DEAE-cellulose that are used for ion exchange chromatography to purify proteins. At pH 8 which chromatographic resin has to be used to separate iso-1-cytochrome c from iso-2- cytochrome c from a lysate of yeast cells? If the proteins are separated by application of a concentration gradient carboxymethyl of NaCl applied to the column, which ion Na® or Cle dis- places the proteins from the binding sites on the chroma- tographic resin? cellulose OH H3C CH3 ΘΝΗ HO HỌC, NH H3C 2-(diethylamino)ethyl cellulose (DEAE cellulose) Which protein iso-1-cytochrome c or iso-2-cytochrome c will be the first to be eluted from the column and why? Both cytochromes in their ferrous form are substrates of yeast cytochrome c peroxidase (CCP) and react optimally with this enzyme at pH 8 forming a Michaelis complex of 1:1 stoichiometry (CcP:cyt c). If the isoionic point of the peroxidase is 5.25, what must be the overall electrostatic charge on the peroxidase enzyme for this reaction to occur, positive or negative?
Biology 2e
2nd Edition
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:Matthew Douglas, Jung Choi, Mary Ann Clark
Chapter6: Metabolism
Section: Chapter Questions
Problem 7RQ: Which of the following comparisons or contrasts between endergonic and exergonic reactions is false?...
Related questions
Question
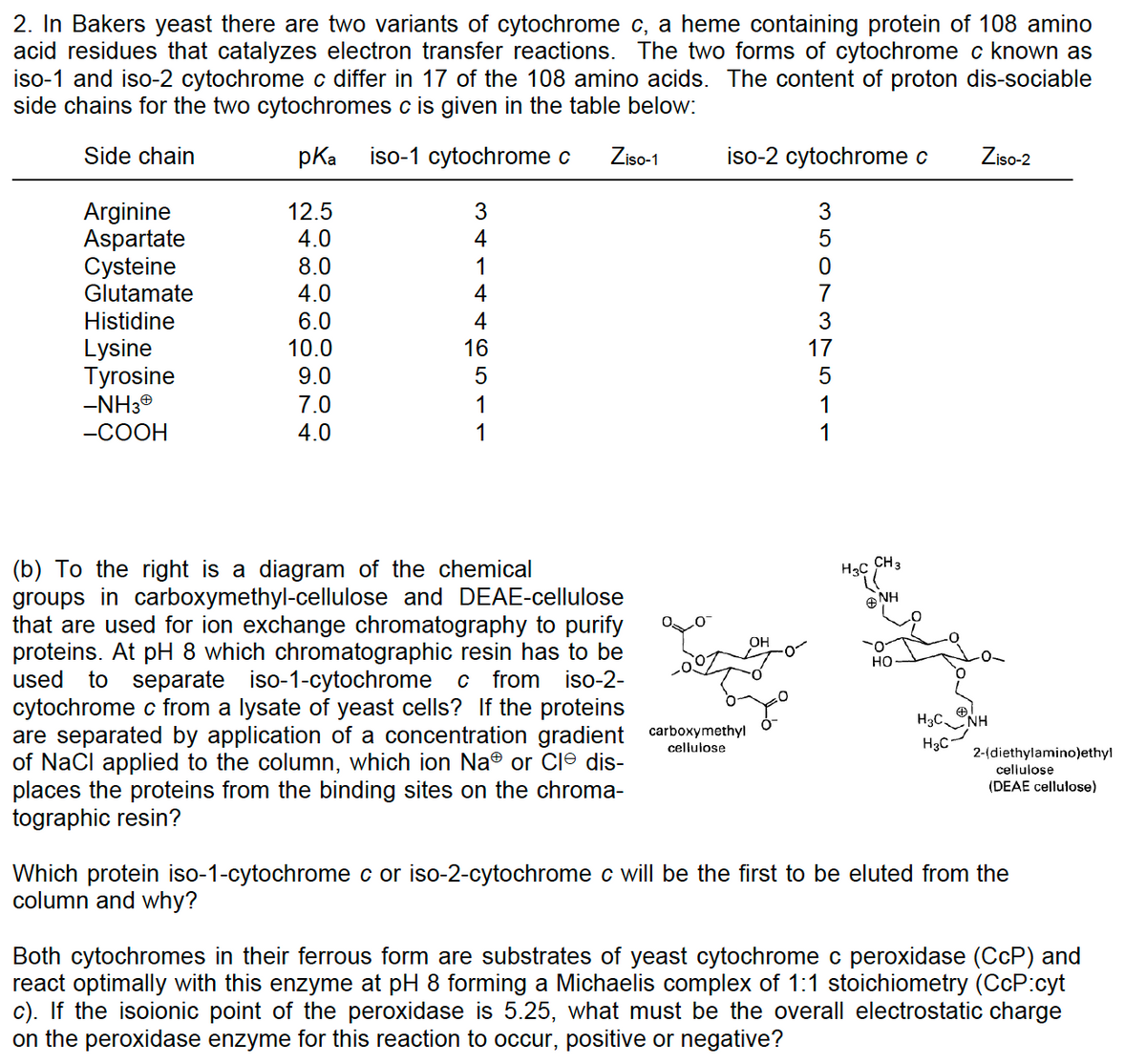
Transcribed Image Text:2. In Bakers yeast there are two variants of cytochrome c, a heme containing protein of 108 amino
acid residues that catalyzes electron transfer reactions. The two forms of cytochrome c known as
iso-1 and iso-2 cytochrome c differ in 17 of the 108 amino acids. The content of proton dis-sociable
side chains for the two cytochromes c is given in the table below:
Side chain
pKa iso-1 cytochrome c Ziso-1
Arginine
Aspartate
Cysteine
Glutamate
Histidine
Lysine
Tyrosine
-NH3Ⓡ
-COOH
12.5
4.0
8.0
4.0
6.0
10.0
9.0
7.0
4.0
34
1
4
4
16
⑤
5
1
1
iso-2 cytochrome c
(b) To the right is a diagram of the chemical
groups in carboxymethyl-cellulose and DEAE-cellulose
that are used for ion exchange chromatography to purify
proteins. At pH 8 which chromatographic resin has to be
used to separate iso-1-cytochrome c from iso-2-
cytochrome c from a lysate of yeast cells? If the proteins
are separated by application of a concentration gradient carboxymethyl
of NaCl applied to the column, which ion Na® or Cle dis-
places the proteins from the binding sites on the chroma-
tographic resin?
Joo
cellulose
OH
3
77751 vouw
5
7
3
H3C
CH 3
NH
HO
Ziso-2
HỌC LẠNH
H3C
2-(diethylamino)ethyl
cellulose
(DEAE cellulose)
Which protein iso-1-cytochrome c or iso-2-cytochrome c will be the first to be eluted from the
column and why?
Both cytochromes in their ferrous form are substrates of yeast cytochrome c peroxidase (CCP) and
react optimally with this enzyme at pH 8 forming a Michaelis complex of 1:1 stoichiometry (CcP:cyt
c). If the isoionic point of the peroxidase is 5.25, what must be the overall electrostatic charge
on the peroxidase enzyme for this reaction to occur, positive or negative?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:
9781305389892
Author:
Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:
Cengage Learning

Biology: The Unity and Diversity of Life (MindTap…
Biology
ISBN:
9781305073951
Author:
Cecie Starr, Ralph Taggart, Christine Evers, Lisa Starr
Publisher:
Cengage Learning

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:
9781305389892
Author:
Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:
Cengage Learning

Biology: The Unity and Diversity of Life (MindTap…
Biology
ISBN:
9781305073951
Author:
Cecie Starr, Ralph Taggart, Christine Evers, Lisa Starr
Publisher:
Cengage Learning

Biology: The Unity and Diversity of Life (MindTap…
Biology
ISBN:
9781337408332
Author:
Cecie Starr, Ralph Taggart, Christine Evers, Lisa Starr
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Anatomy & Physiology
Biology
ISBN:
9781938168130
Author:
Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:
OpenStax College