Suh and Savizky studied the thermodynamics of human a - lactalbumin (HLA) unfolding in the absence (apo) or presence of various metal ions (Suh, J. J. and Savizky, R. M., Int. J. Biophys., 2011, 1(1), 1-6). You may assume that the protein starts out in the folded state and becomes denatured when it unfolds. According to the data below, which metal ion results in the greatest enthalpic stabilization of the folded state? AG (KJ/mol) -5.29 ± 0.81 4.10 ± 0.17 AS (kJ/mol*K) 0.61 ± 0.07 AH (kJ/mol) Experiment Аро-HLA MgCl; + apo-HLA ZNC1, + apo-HLA CaCl + apo-HLA CoCh + apo-HLA MnCl, + apo-HLA SICI, + apo-HLA CaCl + apo-HLA NaCl+ apo-HLA KC1+ apo-HLA 184.92 ± 20.78 174.05 ± 5.72 0.55 ± 0.02 121.84 ± 6.02 3.62 ± 0.13 0.38 ± 0.02 181.79 ± 11.56 6.06 ± 0.46 0.57±0.04 184.69 ± 10.47 10.68 ± 0.44 0.56 ± 0.03 238.65 ± 15.22 14.10 ± 0.88 0.72 ± 0.05 223.41 ± 10.61 15.08 ± 0.91 0.67 0.03 232.16 ± 14.87 19.91 ± 1.33 0.68 ± 0.04 162.17± 6.27 -4.19 ± 0.18 0.54 ± 0.02 154.02 ± 8.73 -3.58 ± 0.24 0.51 ± 0.03 Mn2+ Zn2+ K+ none of these - the enzyme is more stable in the absence of metal ions Co2+
Suh and Savizky studied the thermodynamics of human a - lactalbumin (HLA) unfolding in the absence (apo) or presence of various metal ions (Suh, J. J. and Savizky, R. M., Int. J. Biophys., 2011, 1(1), 1-6). You may assume that the protein starts out in the folded state and becomes denatured when it unfolds. According to the data below, which metal ion results in the greatest enthalpic stabilization of the folded state? AG (KJ/mol) -5.29 ± 0.81 4.10 ± 0.17 AS (kJ/mol*K) 0.61 ± 0.07 AH (kJ/mol) Experiment Аро-HLA MgCl; + apo-HLA ZNC1, + apo-HLA CaCl + apo-HLA CoCh + apo-HLA MnCl, + apo-HLA SICI, + apo-HLA CaCl + apo-HLA NaCl+ apo-HLA KC1+ apo-HLA 184.92 ± 20.78 174.05 ± 5.72 0.55 ± 0.02 121.84 ± 6.02 3.62 ± 0.13 0.38 ± 0.02 181.79 ± 11.56 6.06 ± 0.46 0.57±0.04 184.69 ± 10.47 10.68 ± 0.44 0.56 ± 0.03 238.65 ± 15.22 14.10 ± 0.88 0.72 ± 0.05 223.41 ± 10.61 15.08 ± 0.91 0.67 0.03 232.16 ± 14.87 19.91 ± 1.33 0.68 ± 0.04 162.17± 6.27 -4.19 ± 0.18 0.54 ± 0.02 154.02 ± 8.73 -3.58 ± 0.24 0.51 ± 0.03 Mn2+ Zn2+ K+ none of these - the enzyme is more stable in the absence of metal ions Co2+
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter30: Capillary Electrophoresis, Electrochromatography, And Field-flow Fractionation
Section: Chapter Questions
Problem 30.12QAP
Related questions
Question
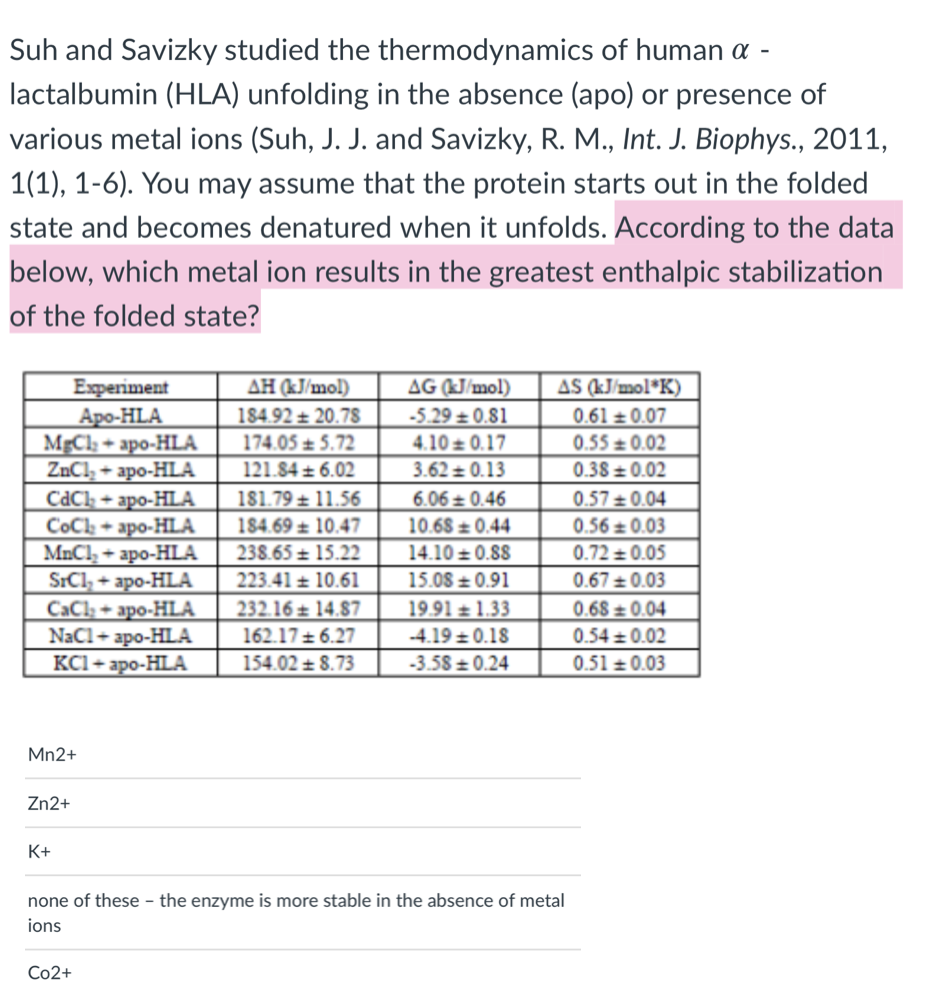
Transcribed Image Text:Suh and Savizky studied the thermodynamics of human a -
lactalbumin (HLA) unfolding in the absence (apo) or presence of
various metal ions (Suh, J. J. and Savizky, R. M., Int. J. Biophys., 2011,
1(1), 1-6). You may assume that the protein starts out in the folded
state and becomes denatured when it unfolds. According to the data
below, which metal ion results in the greatest enthalpic stabilization
of the folded state?
AS (kJ/mol*K)
0.61 ± 0.07
0.55 ± 0.02
AH (kJ/mol)
Experiment
Аро-HLA
MĘC1; + apo-HLA
ZnCl, + apo-HLA
CdCl, + apo-HLA
CoCh + apo-HLA
MnCl, + apo-HLA
SICI, + apo-HLA
CaCl + apo-HLA
- apo-HLA
KC1+ apo-HLA
AG (KJ/mol)
184.92 ± 20.78
-5.29 ± 0.81
4.10 ± 0.17
3.62 0.13
174.05 ± 5.72
121.84 ± 6.02
0.38 ± 0.02
181.79 ± 11.56
6.06 = 0.46
0.57 ±0.04
184.69 ± 10.47
10.68 ± 0.44
0.56 ± 0.03
238.65 ± 15.22
14.10 ± 0.88
0.72 ± 0.05
15.08 ± 0.91
19.91 ± 1.33
223.41 ± 10.61
0.67 +0.03
232.16 ± 14.87
0.68 0.04
NaCl+a
162.17 ± 6.27
-4.19 + 0.18
0.54 + 0.02
154.02 ± 8.73
-3.58 ± 0.24
0.51 + 0.03
Mn2+
Zn2+
K+
none of these - the enzyme is more stable in the absence of metal
ions
Со2+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
