Ch 7 HW Najma Jama - Community College- CHEM 1020 - Spring20 - NGWENDSON > Activities and Due Dates > Ch 7 HW Assignment Score: 42.6% Give Up? O Hint Resources Check Answer Kス < Question 15 of 23 <. A sample of ethanol, C2H6O, has a mass of 12.00 g. Calculate the number of ethanol molecules in the sample. molecules terms of use contact us help about us privacy policy careers 13 59 6. Aa APR PDF 16 DD DII F12 F11 F10 F9 F8 80 F7 F6 F5 F4 F3 F2 & delete earning Ch 7 HW Najma Jama - Normandale Community College - CHEM 1020 - Spring20 - NGWENDSON > Activities and Due Dates > Ch 7 HW ns Assignment Score: 42.6% Resources L Give Up? Hint K: K. Check Answer Correct Question 14 of 23 <> 100% Calculate the mass in grams of 3.32 x 1024 molecules of methanol. The chemical formula for methanol is CH4O. Correct 0% mass: 100% Correct 0% In Progress 0/100 0% arning, Inc. about us privacy policy terms of use contact us help careers 59 6. 13 Dhetioners PAGES APR Aa 16 PDF DII F10 F11 F12 F7 F8 F9 F3 F4 F5 F6 F2 %$4 & delete
Ch 7 HW Najma Jama - Community College- CHEM 1020 - Spring20 - NGWENDSON > Activities and Due Dates > Ch 7 HW Assignment Score: 42.6% Give Up? O Hint Resources Check Answer Kス < Question 15 of 23 <. A sample of ethanol, C2H6O, has a mass of 12.00 g. Calculate the number of ethanol molecules in the sample. molecules terms of use contact us help about us privacy policy careers 13 59 6. Aa APR PDF 16 DD DII F12 F11 F10 F9 F8 80 F7 F6 F5 F4 F3 F2 & delete earning Ch 7 HW Najma Jama - Normandale Community College - CHEM 1020 - Spring20 - NGWENDSON > Activities and Due Dates > Ch 7 HW ns Assignment Score: 42.6% Resources L Give Up? Hint K: K. Check Answer Correct Question 14 of 23 <> 100% Calculate the mass in grams of 3.32 x 1024 molecules of methanol. The chemical formula for methanol is CH4O. Correct 0% mass: 100% Correct 0% In Progress 0/100 0% arning, Inc. about us privacy policy terms of use contact us help careers 59 6. 13 Dhetioners PAGES APR Aa 16 PDF DII F10 F11 F12 F7 F8 F9 F3 F4 F5 F6 F2 %$4 & delete
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.15QAP
Related questions
Question
Transcribed Image Text:Ch 7 HW
Najma Jama -
Community College- CHEM 1020 - Spring20 - NGWENDSON > Activities and Due Dates > Ch 7 HW
Assignment Score:
42.6%
Give Up?
O Hint
Resources
Check Answer
Kス
< Question 15 of 23
<.
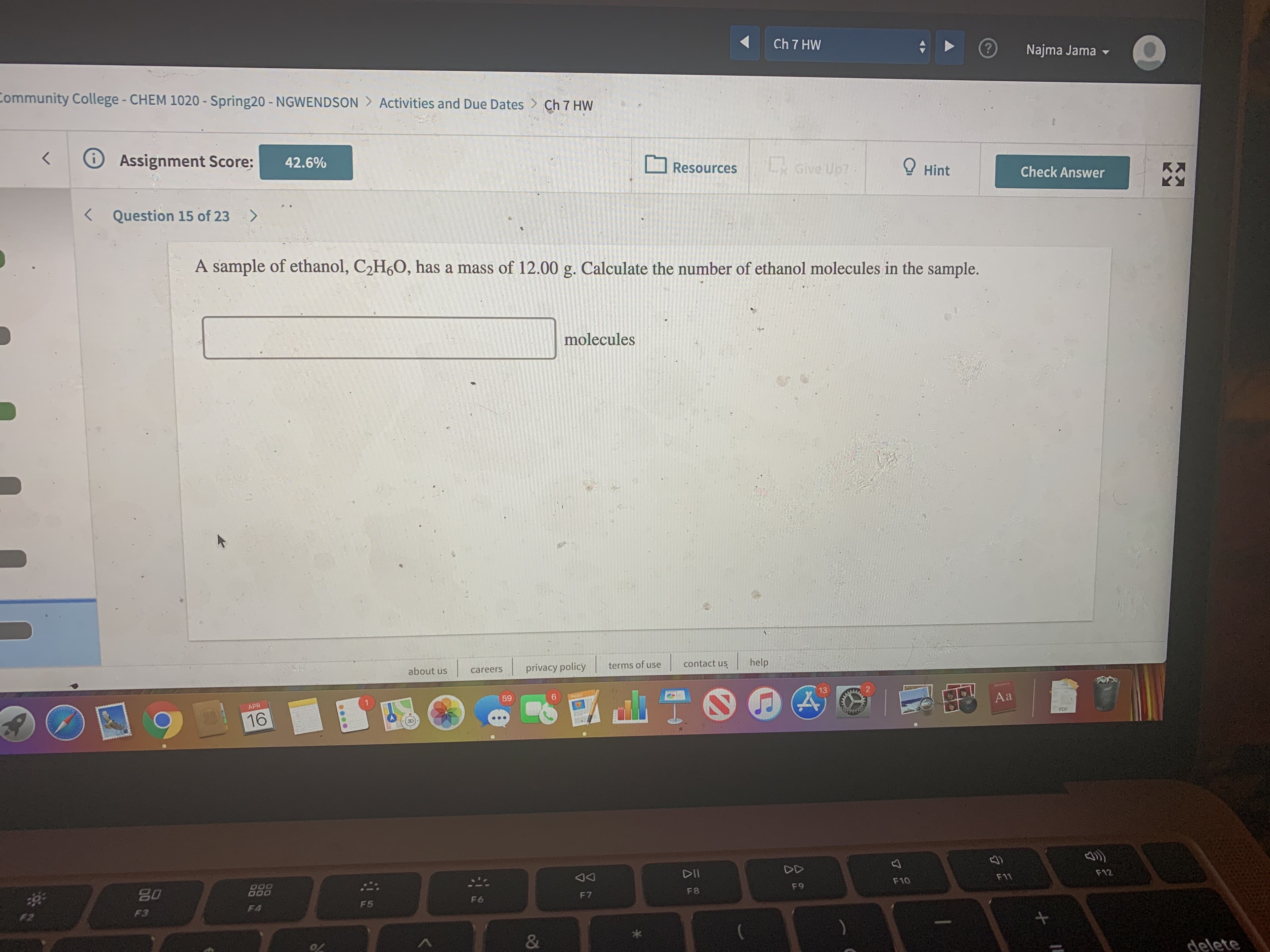
A sample of ethanol, C2H6O, has a mass of 12.00 g. Calculate the number of ethanol molecules in the sample.
molecules
terms of use
contact us
help
about us
privacy policy
careers
13
59
6.
Aa
APR
PDF
16
DD
DII
F12
F11
F10
F9
F8
80
F7
F6
F5
F4
F3
F2
&
delete
Transcribed Image Text:earning
Ch 7 HW
Najma Jama -
Normandale Community College - CHEM 1020 - Spring20 - NGWENDSON > Activities and Due Dates > Ch 7 HW
ns
Assignment Score:
42.6%
Resources
L Give Up?
Hint
K:
K.
Check Answer
Correct
Question 14 of 23
<>
100%
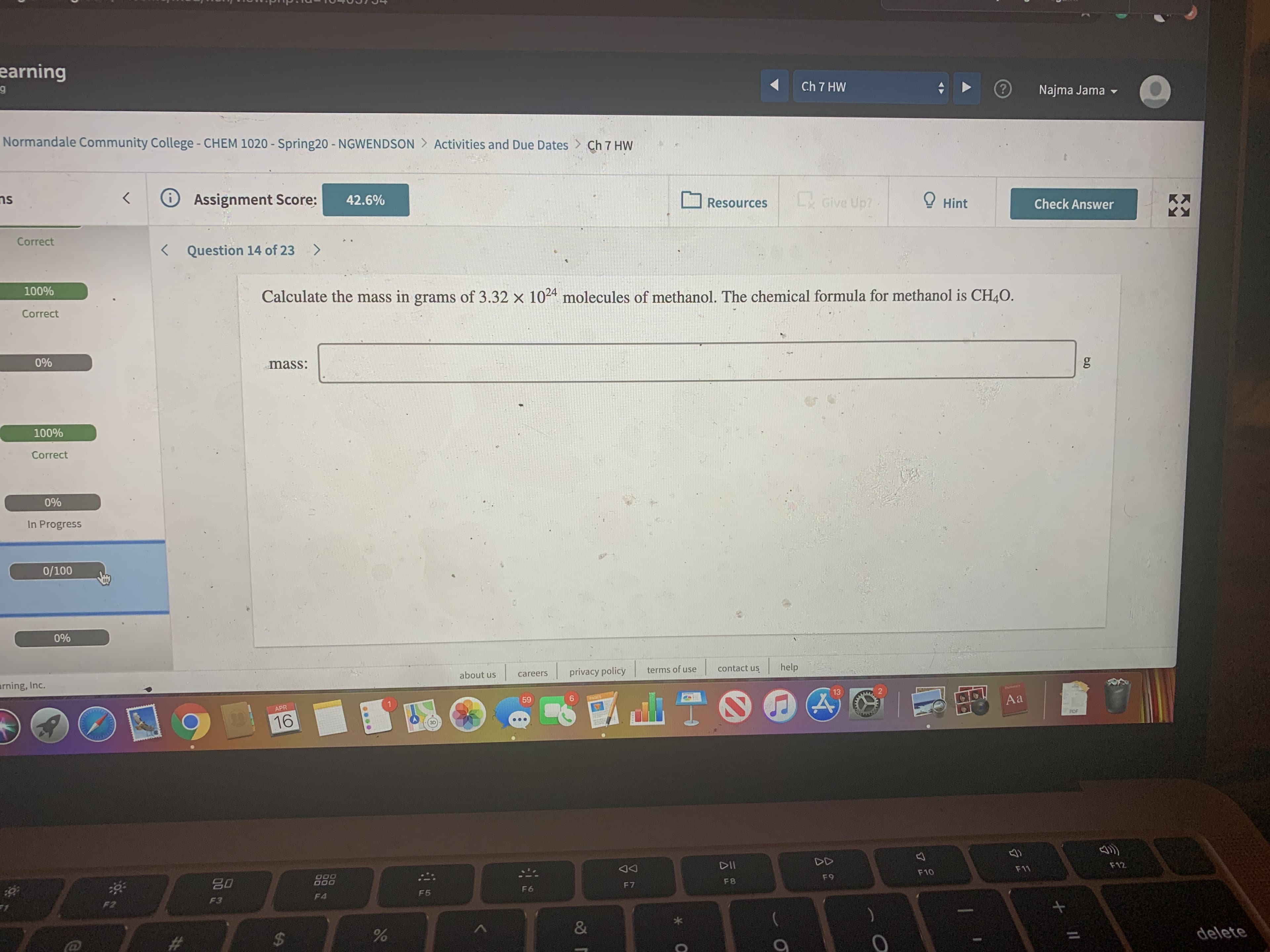
Calculate the mass in grams of 3.32 x 1024 molecules of methanol. The chemical formula for methanol is CH4O.
Correct
0%
mass:
100%
Correct
0%
In Progress
0/100
0%
arning, Inc.
about us
privacy policy
terms of use
contact us
help
careers
59
6.
13
Dhetioners
PAGES
APR
Aa
16
PDF
DII
F10
F11
F12
F7
F8
F9
F3
F4
F5
F6
F2
%$4
&
delete
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax