Cooling curve of pure naphthalene Cooling graph of naphthalene and sulfur mixture 100 90 70 60 Freezing point 73°C 75 50 40 -Tenperture o 30 70 20 10 65 100 150 200 100 200 300 400 Time, t(second) t (s) Freezing point of pure naphthalene T1 =...80..C Freezing point of solution T2 =73.C Freezing point of depression from graph AT =7. C Weight of naphthalene= 5,028g Weight of Sulfur-1,011g Kefor naphthalene= 6,8 °C/m %3D Temperature ("C)
Cooling curve of pure naphthalene Cooling graph of naphthalene and sulfur mixture 100 90 70 60 Freezing point 73°C 75 50 40 -Tenperture o 30 70 20 10 65 100 150 200 100 200 300 400 Time, t(second) t (s) Freezing point of pure naphthalene T1 =...80..C Freezing point of solution T2 =73.C Freezing point of depression from graph AT =7. C Weight of naphthalene= 5,028g Weight of Sulfur-1,011g Kefor naphthalene= 6,8 °C/m %3D Temperature ("C)
Chapter84: Fractional Distillation, Azeotropes
Section: Chapter Questions
Problem 5P
Related questions
Question
2.Calculate the molecular weight of sulfur by using m, W1and W2
3.The atomic weight of sulfur is 32 g/mol. By using the result from (2), write the molecular formula of sulfur as Sn. What is n?
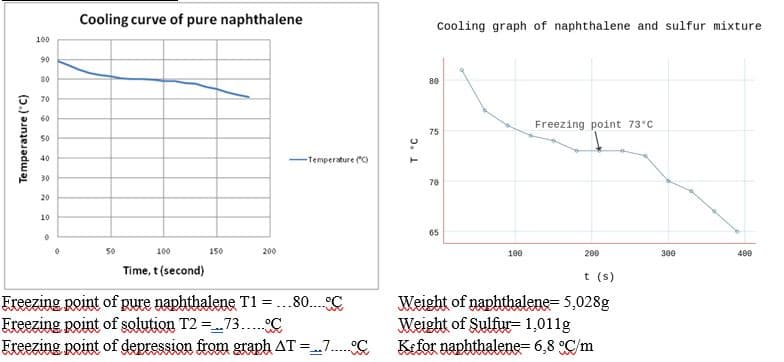
Transcribed Image Text:Cooling curve of pure naphthalene
Cooling graph of naphthalene and sulfur mixture
100
90
80
80
70
60
Freezing point 73°C
75
50
40
-Temperature C)
30
70
20
10
65
50
100
150
200
100
200
300
400
Time, t (second)
t (s)
Ereezing point of pure naphthalene T1 =...80.. C
Freezing point of solution T2 =73.. °C
Ereezing point of depression from graph AT =7. C
Weight of naphthalene= 5,028g
Weight of Sulfur= 1,011g
Kefor naphthalene= 6,8 °C/m
Temperature (°C)

Transcribed Image Text:2.Calculate the molecular weight of sulfur by using m, Wland W2
Name:
Group No:
Department:
3. The atomic weight of sulfur is 32 g/mol. By using the result from (2), write the molecular
formula of sulfur as Sn. What is n?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,