Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter21: Structure And Bonding In Solids
Section: Chapter Questions
Problem 36P
Related questions
Question
Experimentally, it is found that the value of DO varies with the chemical nature of the ligand according to the spectrochemical series: S2− < Cl− < OH− ≈ RCO2− < H2O ≈ RS− < NH3 ≈ imidazole (the side chain of histidine) < CN− < CO. (a) Draw an energy level diagram like those in Fig. 10.41 showing the configuration of the d electrons on the metal ion in [Fe(OH2)6]3+ and [Fe(CN)6]3−.
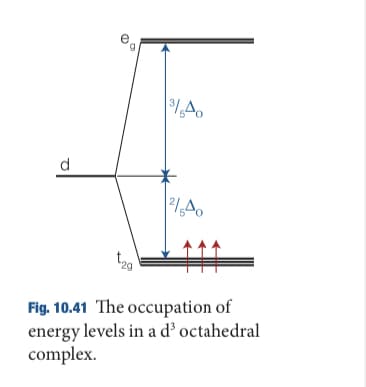
Transcribed Image Text:d.
Fig. 10.41 The occupation of
energy levels in a d’ octahedral
complex.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

