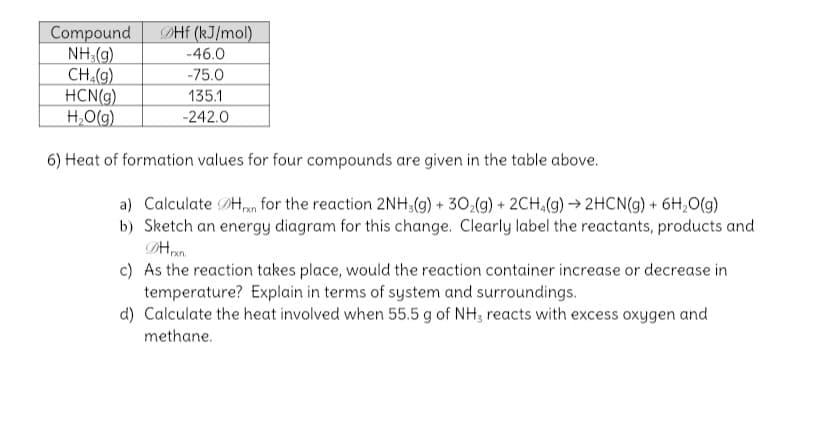
DHE (kJ/mol) Compound NH (g) CH.(9) HCN(g) H,O(g) -46.0 -75.0 135.1 -242.0 6) Heat of formation values for four compounds are given in the table above. a) Calculate DHn for the reaction 2NH;(g) + 30,(g) + 2CH.(9) → 2HCN(g) + 6H,O(g)
DHE (kJ/mol) Compound NH (g) CH.(9) HCN(g) H,O(g) -46.0 -75.0 135.1 -242.0 6) Heat of formation values for four compounds are given in the table above. a) Calculate DHn for the reaction 2NH;(g) + 30,(g) + 2CH.(9) → 2HCN(g) + 6H,O(g)
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 5.34QE
Related questions
Question
Transcribed Image Text:DHF (kJ/mol)
Compound
NH:(g)
CH.(g)
HCN(g)
H,O(g)
-46.0
-75.0
135.1
-242.0
6) Heat of formation values for four compounds are given in the table above.
a) Calculate DHpn for the reaction 2NH;(g) + 30,(g) + 2CH,(9) → 2HCN(9) + 6H,O(g)
b) Sketch an energy diagram for this change. Clearly label the reactants, products and
c) As the reaction takes place, would the reaction container increase or decrease in
temperature? Explain in terms of system and surroundings.
d) Calculate the heat involved when 55.5 g of NH; reacts with excess oxygen and
methane.
Expert Solution
Step 1
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax