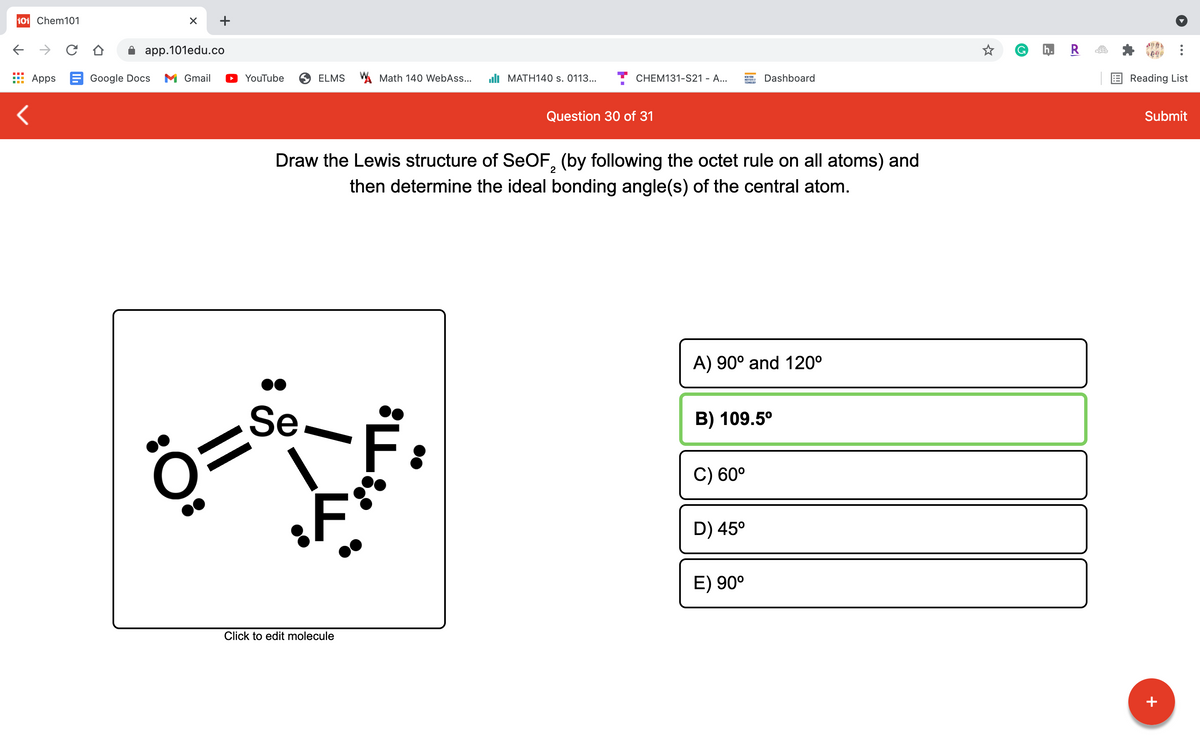
Draw the Lewis structure of SEOF, (by following the octet rule on all atoms) and then determine the ideal bonding angle(s) of the central atom. A) 90° and 120° Se. B) 109.5° C) 60° D) 45° E) 90° Click to edit molecule
Draw the Lewis structure of SEOF, (by following the octet rule on all atoms) and then determine the ideal bonding angle(s) of the central atom. A) 90° and 120° Se. B) 109.5° C) 60° D) 45° E) 90° Click to edit molecule
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter4: Molecular Structure And Orbitals
Section: Chapter Questions
Problem 33E: Write Lewis structures and predict the molecular structures of the following. (See Exercises 25 and...
Related questions
Question
The structure is wrong, can someone help me get the right structure?
Transcribed Image Text:101 Chem101
+
app.101edu.co
G
h. R
Apps E Google Docs
M Gmail
O YouTube
ELMS Math 140 WebAss...
uli MATH140 s. 0113...
CHEM131-S21 - A...
Dashboard
Reading List
TECNOLDY
Question 30 of 31
Submit
Draw the Lewis structure of SEOF, (by following the octet rule on all atoms) and
2
then determine the ideal bonding angle(s) of the central atom.
A) 90° and 120°
Se
B) 109.5°
C) 60°
D) 45°
E) 90°
Click to edit molecule
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning