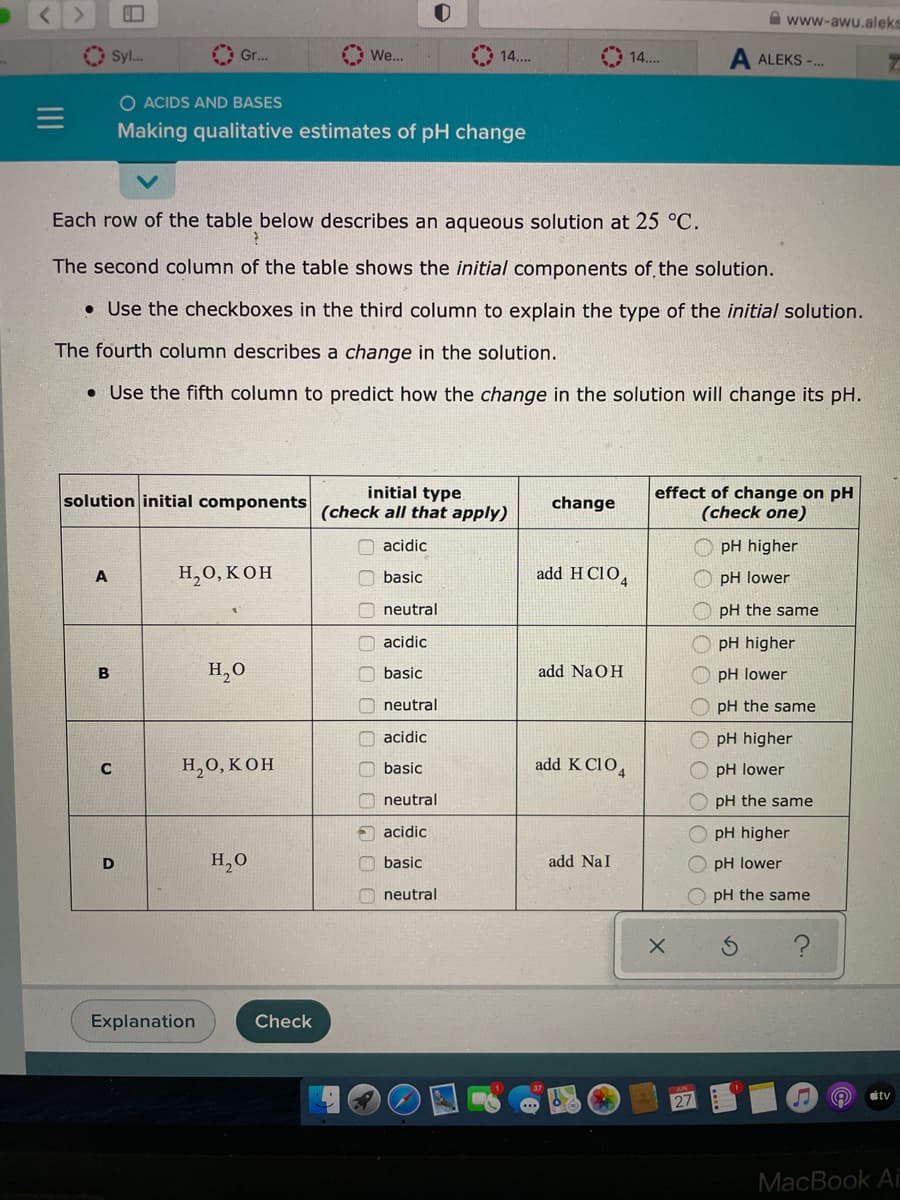
Each row of the table below describes an aqueous solution at 25 °C. ? The second column of the table shows the initial components of the solution. • Use the checkboxes in the third column to explain the type of the initial solution. The fourth column describes a change in the solution. . Use the fifth column to predict how the change in the solution will change its pH. solution initial components A B C D H₂O, KOH H₂O H₂O, KOH Explanation H₂O Check initial type (check all that apply) acidic basic neutral acidic basic neutral acidic basic neutral acidic basic neutral change add HC104 add NaOH add K CIO4 add Nal effect of change on pH (check one) X 00 00 pH higher pH lower pH the same pH higher pH lower pH the same pH higher pH lower pH the same pH higher pH lower pH the same S ?
Each row of the table below describes an aqueous solution at 25 °C. ? The second column of the table shows the initial components of the solution. • Use the checkboxes in the third column to explain the type of the initial solution. The fourth column describes a change in the solution. . Use the fifth column to predict how the change in the solution will change its pH. solution initial components A B C D H₂O, KOH H₂O H₂O, KOH Explanation H₂O Check initial type (check all that apply) acidic basic neutral acidic basic neutral acidic basic neutral acidic basic neutral change add HC104 add NaOH add K CIO4 add Nal effect of change on pH (check one) X 00 00 pH higher pH lower pH the same pH higher pH lower pH the same pH higher pH lower pH the same pH higher pH lower pH the same S ?
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter16: Acids And Bases
Section: Chapter Questions
Problem 51A
Related questions
Question
100%
Transcribed Image Text:Syl...
A
solution initial components
B
C
Gr...
D
O ACIDS AND BASES
Making qualitative estimates of pH change
Each row of the table below describes an aqueous solution at 25 °C.
The second column of the table shows the initial components of the solution.
. Use the checkboxes in the third column to explain the type of the initial solution.
The fourth column describes a change in the solution.
. Use the fifth column to predict how the change in the solution will change its pH.
H2O, KOH
Explanation
H₂O
H2O, KOH
We...
H₂O
Check
O
14....
OO
initial type
(check all that apply)
acidic
basic
neutral
acidic
basic
neutral
acidic
basic
neutral
acidic
basic
neutral
change
add HClO4
add NaOH
add K C104
add Nal
14....
X
X
effect of change on pH
(check one)
ООС
0 0 0/0 О С
www-awu.aleks
A ALEKS-...
27
pH higher
pH lower
pH the same
pH higher
pH lower
pH the same
pH higher
pH lower
OpH the same
pH higher
pH
lower
pH the same
S
n
Ⓡ
tv
MacBook Ai
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning