The figures below show the most common appearance of matter that we recognize. Recall that particles that make up matter behaves in a very specific way that gives the material its distinct property, such as a definite structure or taking the shape of its container. Can you guess which states of matter is being represented by the particle arrangements shown below? Defend your answer. This is most likely a: Because: twinkt.com Figure A twinkt.com Figure B twinkl.com Figure C
The figures below show the most common appearance of matter that we recognize. Recall that particles that make up matter behaves in a very specific way that gives the material its distinct property, such as a definite structure or taking the shape of its container. Can you guess which states of matter is being represented by the particle arrangements shown below? Defend your answer. This is most likely a: Because: twinkt.com Figure A twinkt.com Figure B twinkl.com Figure C
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter1: The Atom In Modern Chemistry
Section: Chapter Questions
Problem 16P: In the problem 15 above, what is vy , the y-component of the electron’s velocity, when it has...
Related questions
Question
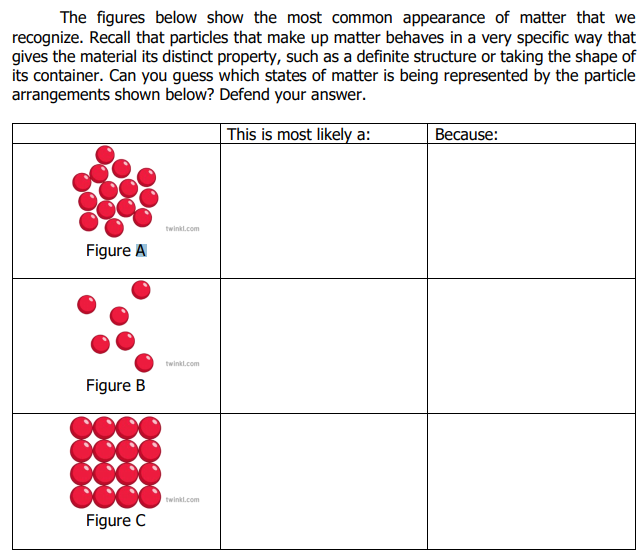
Transcribed Image Text:The figures below show the most common appearance of matter that we
recognize. Recall that particles that make up matter behaves in a very specific way that
gives the material its distinct property, such as a definite structure or taking the shape of
its container. Can you guess which states of matter is being represented by the particle
arrangements shown below? Defend your answer.
This is most likely a:
Because:
twinkl.com
Figure A
twinkl.com
Figure B
twinkl.com
Figure C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning