Erwin Schrödinger Use the References to access important values if needed for this question. Orbitals which result from solving the Schrödinger wave equation can be represented by electron "cloud" pictures. The shapes of the orbitals are distinguished by their nodes, places where the electron density equals zero. Based upon the number of spherical and angular nodes, label the orbitals below as 1s, 2p, etc. Specify the value of n and / for each: This is a orbital. n = and / = This is a n= orbital. and / = This is a orbital. n = and / =
Erwin Schrödinger Use the References to access important values if needed for this question. Orbitals which result from solving the Schrödinger wave equation can be represented by electron "cloud" pictures. The shapes of the orbitals are distinguished by their nodes, places where the electron density equals zero. Based upon the number of spherical and angular nodes, label the orbitals below as 1s, 2p, etc. Specify the value of n and / for each: This is a orbital. n = and / = This is a n= orbital. and / = This is a orbital. n = and / =
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter5: Quantum Mechanics And Atomic Structure
Section: Chapter Questions
Problem 9P: Use the mathematical expression for the 2pz wave function of a one-electron atom (see Table 5.2) to...
Related questions
Question
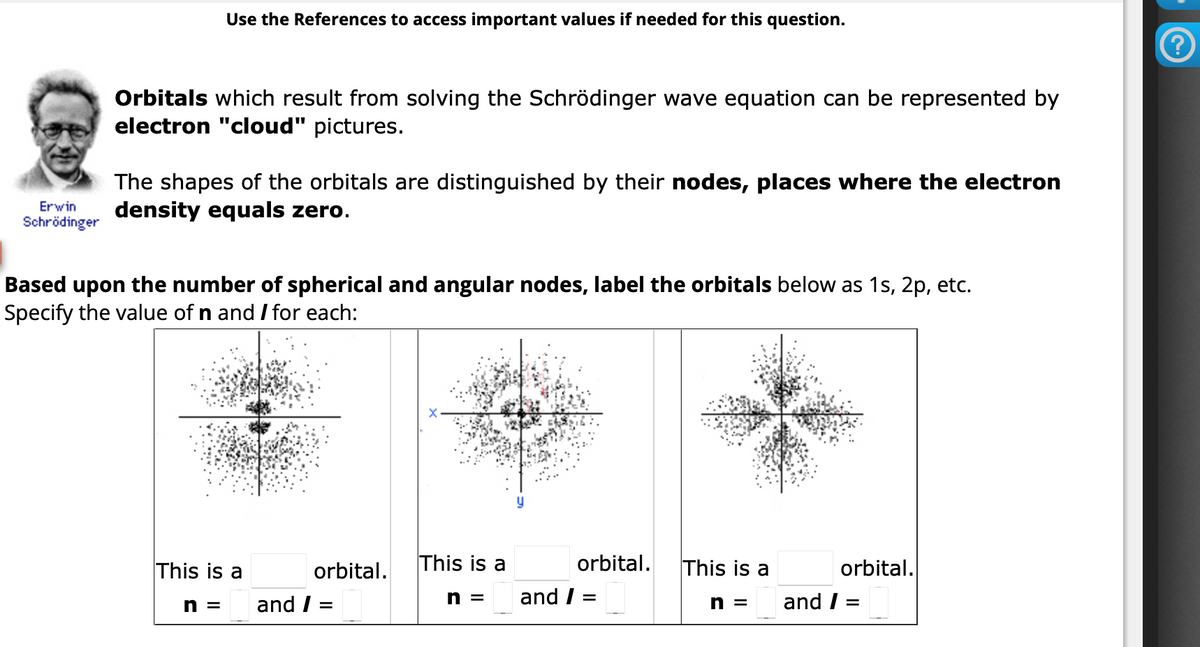
Transcribed Image Text:Erwin
Schrödinger
Use the References to access important values if needed for this question.
Orbitals which result from solving the Schrödinger wave equation can be represented by
electron "cloud" pictures.
The shapes of the orbitals are distinguished by their nodes, places where the electron
density equals zero.
Based upon the number of spherical and angular nodes, label the orbitals below as 1s, 2p, etc.
Specify the value of n and I for each:
This is a
n = and /
orbital.
=
This is a
n =
orbital.
and / =
This is a
n =
and /
orbital.
=
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning