
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Need help with Part 4
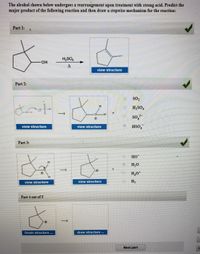
Transcribed Image Text:The alcohol shown below undergoes a rearrangement upon treatment with strong acid. Predict the
major product of the following reaction and then draw a stepwise mechanism for the reaction:
Part 1:
H,SO4
view structure
Part 2:
SO2
H,SO,
SO
so,-
view structure
view structure
HSO,
Part 3:
но
H,0
H,0*
view structure
view structure
0 H2
Part 4 out of 5
finish structure ..
draw structure ...
Next part
+.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Figure 3-1 1 А. СНЗ с. CHz CH3 CH-CH-CH3 CH ₂ CH 3 в. н D. CH CH3 . тоен - сненсне енз СнЗarrow_forwardArrange the compounds by boiling point. 80 F3 $ R hexane: H₂C-CH₂-CH₂-CH₂-CH₂-CH3 pentane: H₂C-CH₂-CH₂-CH₂-CH3 000 000 F4 % T F5 A MacBook Pro Y & 7 o F7 U Highest boiling point Lowest boiling point Answer Bank propane: H₂C-CH₂-CH3 * 00 8 DII F8 - ( 9 F9 ) 0 F10 P F11arrow_forwardyout References 2 v Font ₂x²A - A V Mailings Review View Help A A Aa AEE ALT Y f3 5 United States) Text Predictions: On O 14 101 === Paragraph Search (Alt+Q) 4.All are correct V Accessibility: Good to go 15 5 1.To the polarity of water and that organic compounds are nonpolar. f6 D Normal Organic compounds tend not to mix with water. What is the reason for this chemical characteristic of organic compounds with water? 2.Because water forms a hydrogen bond and hydrocarbons do not have polarity for this, unless they have an oxygen in the chain. 3.Some alkanes can mix with water if they have a carbonyl or hydroxyl group. No Spacing f7 40 Styles hp 18 Heading 1 19 0 0 f10 F17 5 F12 Find Replac Select- Editing 82°F Mosarrow_forward
- 24.34 Draw the product of each of the following reactions. piease show Allarrtw pasheng wedranonsarrow_forwardNEITEHR OF THE ANSWERS SHOULD BE PSI -60 PLEASE IF YOU PUT THAT I WILL DISLIKE AND If you copy ur answers from other bartleby answers i will dislikearrow_forwardHO OH EtOEt cat H2SO4arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY