For 14 reactions from this experiment in which there is a driving force, give the balanced molecular, ionic and net ionic equations. Then identify the driving force for each reaction. Be sure to include the proper state symbols (e.g., aq, s, I, or g) for all substances. For each type of driving force, be sure to include at least one example (e.g., precipitation, gas formation, neutralization).
For 14 reactions from this experiment in which there is a driving force, give the balanced molecular, ionic and net ionic equations. Then identify the driving force for each reaction. Be sure to include the proper state symbols (e.g., aq, s, I, or g) for all substances. For each type of driving force, be sure to include at least one example (e.g., precipitation, gas formation, neutralization).
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
100%
For 14 reactions from this experiment in which there is a driving force, give the balanced molecular, ionic and net ionic equations. Then identify the driving force for each reaction. Be sure to include the proper state symbols (e.g., aq, s, I, or g) for all substances. For each type of driving force, be sure to include at least one example (e.g., precipitation, gas formation, neutralization).

Transcribed Image Text:UC(ag) t Nac H
eMy
ICE
11. Reactants:
Molecular equation
lonic equation
Net ionic equation
Driving force
ti
12. Reactants:
Molecular equation
lonic equation
Net ionic equation
Driving force
13. Reactants:
Molecular equation
lonic equation
Net ionic equation
Driving force
14. Reactants:
Molecular equation
lonic equation
Net ionic equation
Driving force
6.
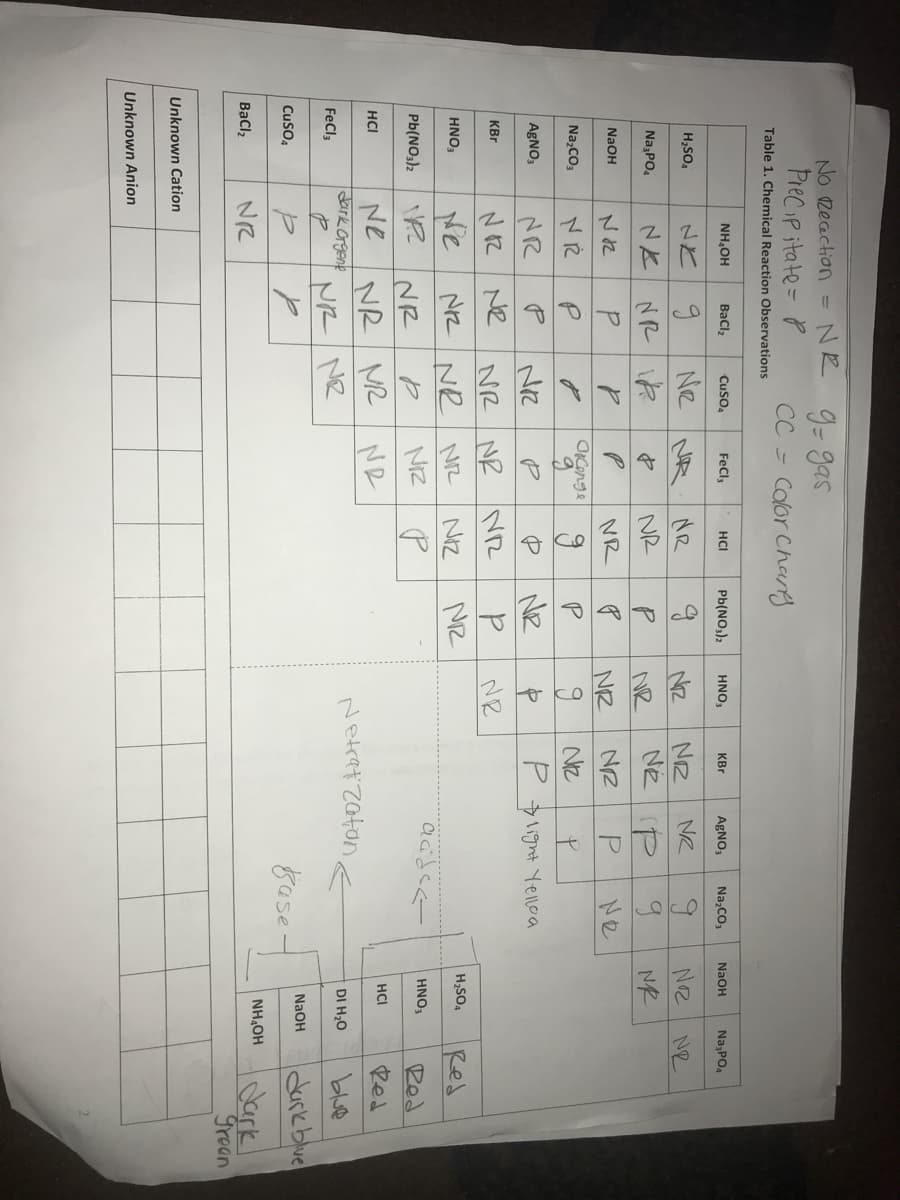
Transcribed Image Text:にko后
No zecaction =
NE 9-gas
Precip itate= P
CC = Calor Chag
Table 1. Chemical Reaction Observations
NH,OH
Bacl,
Cuso,
FeCl,
HCI
Pb(NO,)2
HNO,
KBr
AgNO,
Na,CO,
NAOH
Na,PO,
Ne NR
H,SO,
NZ
NE P
Ne
NR
NK
NR
Na PO,
NR
NR
P.
Ne
NaOH
Oconge
Na,CO3
Ne
NR NR NR
MZ
P 1ight Yene a
NR
NR Ne
AgNO3
NR
KBr
Ne NZ NR NR
NZ
H,SO4
NIZ
Red
HNO;
HNO3
Pb(NO3)2
NR
Red
Red
Ne NR MR Ne
dark Crgene
NR NR
HCI
HCI
Neket Zaton e
DI H,0
blue
FeCl3
dark blue
NaOH
8ase
Cuso4
Iolark
Trean
NH,OH
Bạcl,
NR
Unknown Cation
Unknown Anion
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY