Table 1 Solubility Rules for lonic Compounds Soluble in Water Insoluble in Water Any salt with Li*, Na*, K*, NH4*, NO3- Most chlorides, Cl- AgCI, PbCl2, and Hg2Cl2 Most sulfates, SO2- BaSO4, PBSO4, and CaSO4 Salts with OH-, CO32²-, S², PO43- a. AGNO3 (aq) + NaCl (aq) → b. K3PO4 (aq) + NaOH (aq) – c. (NH4)2SO4 (aq) + Ba(NO3)2 (aq) – d. Au2(SO4)3 (aq) + PbCl2 (aq) e. HgSO4 (aq) + CaCl2 (aq) -
Table 1 Solubility Rules for lonic Compounds Soluble in Water Insoluble in Water Any salt with Li*, Na*, K*, NH4*, NO3- Most chlorides, Cl- AgCI, PbCl2, and Hg2Cl2 Most sulfates, SO2- BaSO4, PBSO4, and CaSO4 Salts with OH-, CO32²-, S², PO43- a. AGNO3 (aq) + NaCl (aq) → b. K3PO4 (aq) + NaOH (aq) – c. (NH4)2SO4 (aq) + Ba(NO3)2 (aq) – d. Au2(SO4)3 (aq) + PbCl2 (aq) e. HgSO4 (aq) + CaCl2 (aq) -
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter24: The Standardization Of A Basic Solution And The Determination Of The Molar Mass Of An Acid
Section: Chapter Questions
Problem 3ASA: A 0.3012g sample of an unknown monoprotic acid requires 24.13mL of 0.0944MNaOH for neutralization to...
Related questions
Question
How do I predict whether each of the reactions below will produce a precipitate and, if so, what would the precipitate be. If there is more than one precipitate, what are both of them. Or if there is no precipitate, state ‘none.’
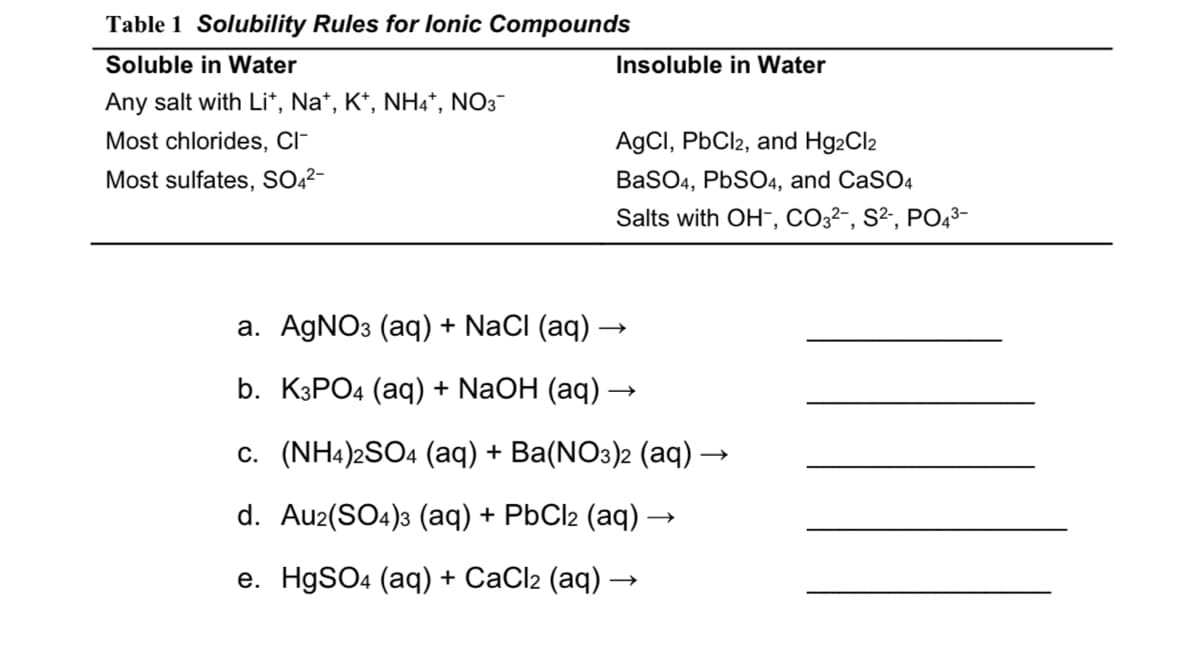
Transcribed Image Text:Table 1 Solubility Rules for lonic Compounds
Soluble in Water
Insoluble in Water
Any salt with Li*, Na*, K*, NH4*, NO3-
Most chlorides, CI-
AgCI, PbCl2, and Hg2Cl2
Most sulfates, SO,²-
BaSO4, PBSO4, and CaSO4
Salts with OH-, CO3²-, S², PO43-
a. AGNO3 (aq) + NaCI (aq)
b. КЗРО4 (аq) + NaOH (aq) —
c. (NH4)2SO4 (aq) + Ba(NO3)2 (aq) -
d. Au2(SO4)3 (aq) + PbCl2 (aq) ·
е. HgSOa (aq) + СaClz (aq).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole



Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole



Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning