For the equation below at equilibrium, what is the shift in the equilibrium position upon adding sodium acetate to the equilibrium mixture of acetic acid and methyl orange, where HMO represents the protonated (acid) form of the indicator and MO- represents the deprotonated (or base) form of the indicator. What is the resulting color of the solution? HMO(aq) + H,O(1) H,O-(aq) + MO(aq) O to the left/red O to the right/yellow O to the left/yellow to the right/red
For the equation below at equilibrium, what is the shift in the equilibrium position upon adding sodium acetate to the equilibrium mixture of acetic acid and methyl orange, where HMO represents the protonated (acid) form of the indicator and MO- represents the deprotonated (or base) form of the indicator. What is the resulting color of the solution? HMO(aq) + H,O(1) H,O-(aq) + MO(aq) O to the left/red O to the right/yellow O to the left/yellow to the right/red
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter16: Solubility And Precipitation Equilibria
Section: Chapter Questions
Problem 70AP
Related questions
Question
100%
i hope you can answer the questions. really need it.

Transcribed Image Text:For the equation below at equilibrium, what is the shift in the equilibrium position
upon adding sodium acetate to the equilibrium mixture of acetic acid and methyl
orange, where HMO represents the protonated (acid) form of the indicator and
MO- represents the deprotonated (or base) form of the indicator. What is the
resulting color of the solution?
HMO(aq)
+ H,O(1) = H.O-(aq) + M0(aq)
O to the left/red
O to the right/yellow
O to the left/yellow
O to the right/red
For the equation below at equilibrium what is the shift in the eguilibrium nonitir
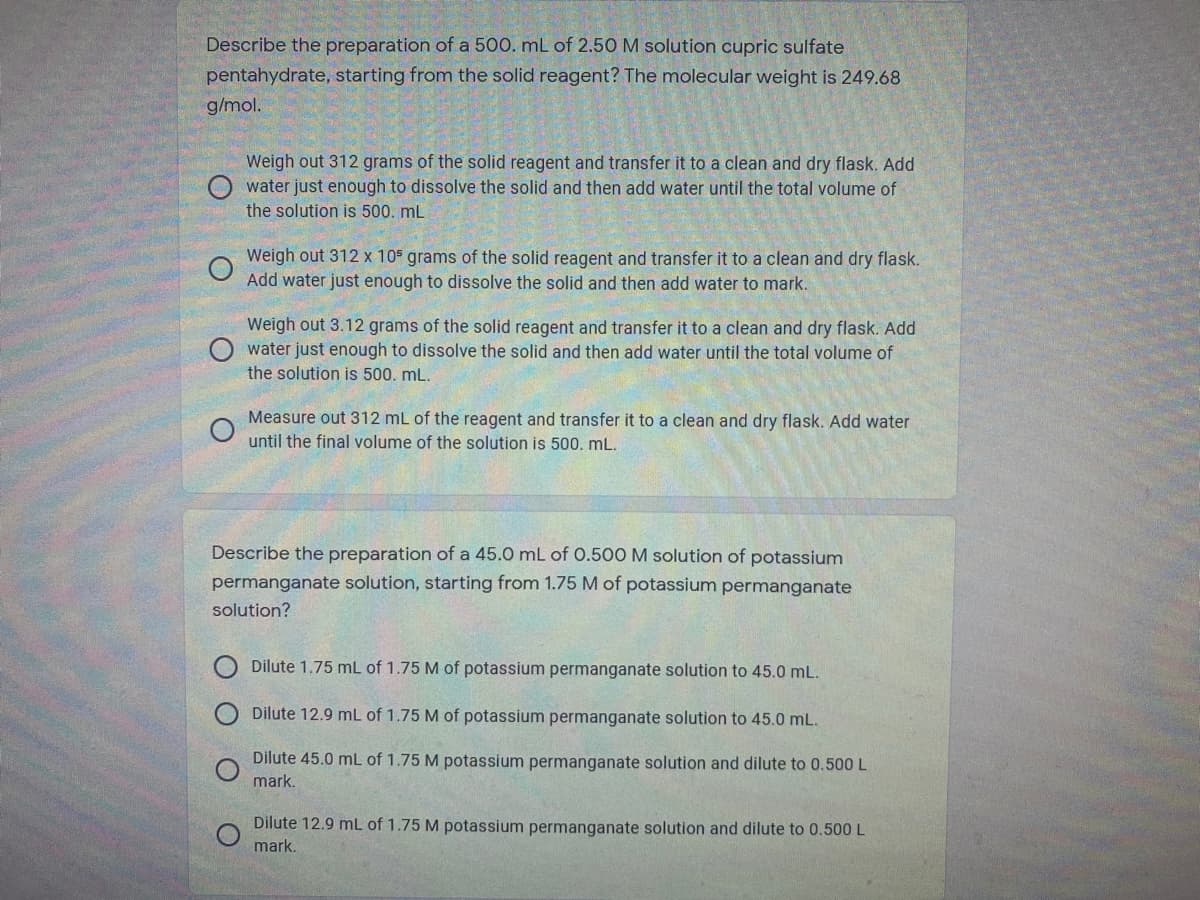
Transcribed Image Text:Describe the preparation of a 500. mL of 2.50 M solution cupric sulfate
pentahydrate, starting from the solid reagent? The molecular weight is 249.68
g/mol.
Weigh out 312 grams of the solid reagent and transfer it to a clean and dry flask. Add
O water just enough to dissolve the solid and then add water until the total volume of
the solution is 500. mL
Weigh out 312 x 105 grams of the solid reagent and transfer it to a clean and dry flask.
Add water just enough to dissolve the solid and then add water to mark.
Weigh out 3.12 grams of the solid reagent and transfer it to a clean and dry flask. Add
O water just enough to dissolve the solid and then add water until the total volume of
the solution is 500. mL.
Measure out 312 mL of the reagent and transfer it to a clean and dry flask. Add water
until the final volume of the solution is 500. mL.
Describe the preparation of a 45.0 mL of 0.500 M solution of potassium
permanganate solution, starting from 1.75 M of potassium permanganate
solution?
Dilute 1.75 mL of 1.75 M of potassium permanganate solution to 45.0 mL.
Dilute 12.9 mL of 1.75 M of potassium permanganate solution to 45.0 mL.
Dilute 45.0 mL of 1.75 M potassium permanganate solution and dilute to 0.500 L
mark.
Dilute 12.9 mL of 1.75 M potassium permanganate solution and dilute to 0.500 L
mark.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,