For the following question, you may need to refer to the table give below. Nucleus I Natural abundance (%) 1H 1/2 100 13C 1/2 1.1 31p 1/2 100 195Pt 1/2 33.8 All other isotopes of C, Pt & Hg are assumed to have I = 0. Shown below 1H NMR spectrum of dimethylphosphine, Me2PH, which is capal
For the following question, you may need to refer to the table give below. Nucleus I Natural abundance (%) 1H 1/2 100 13C 1/2 1.1 31p 1/2 100 195Pt 1/2 33.8 All other isotopes of C, Pt & Hg are assumed to have I = 0. Shown below 1H NMR spectrum of dimethylphosphine, Me2PH, which is capal
Chapter12: Structure Determination: Mass Spectrometry And Infrared Spectroscopy
Section12.SE: Something Extra
Problem 50AP
Related questions
Question
Based on the given information, need to identify which hydrogen atom environments give rise to each of the two signals (at 3.1 ppm and 1.1 ppm). Thank you :)
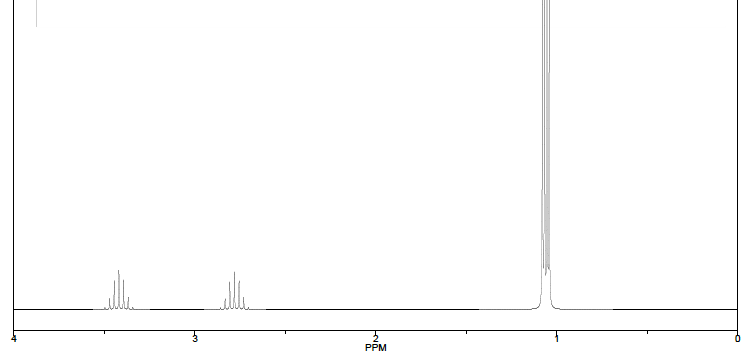
Transcribed Image Text:PPM
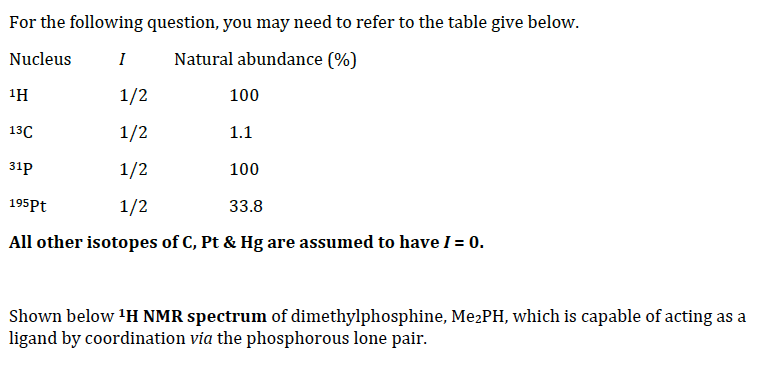
Transcribed Image Text:For the following question, you may need to refer to the table give below.
Nucleus
I
Natural abundance (%)
1H
1/2
100
13C
1/2
1.1
31p
1/2
100
195Pt
1/2
33.8
All other isotopes of C, Pt & Hg are assumed to have I = 0.
Shown below H NMR spectrum of dimethylphosphine, MezPH, which is capable of acting as a
ligand by coordination via the phosphorous lone pair.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning