For the following reaction, 6.92 grams of carbon (graphite) are mixed with excess oxygen gas . The reaction yields 16.5 grams of carbon dioxide carbon (graphite) (s) + oxygen ( g ) → carbon dioxide ( g ) What is the theoretical yield of carbon dioxide ? grams What is the percent yield for this reaction ? Submit Answer Try Another Version 3 item attempts remaining
For the following reaction, 6.92 grams of carbon (graphite) are mixed with excess oxygen gas . The reaction yields 16.5 grams of carbon dioxide carbon (graphite) (s) + oxygen ( g ) → carbon dioxide ( g ) What is the theoretical yield of carbon dioxide ? grams What is the percent yield for this reaction ? Submit Answer Try Another Version 3 item attempts remaining
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter10: Quantity Relationships In Chemical Reactions
Section: Chapter Questions
Problem 70E: Classify each of the following statements as true or false: a Coefficients in a chemical equation...
Related questions
Question
Please provide neat solution
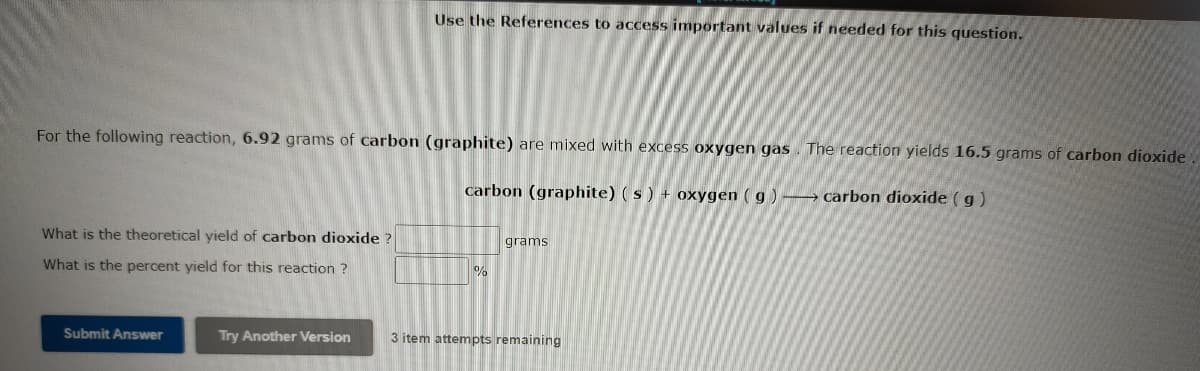
Transcribed Image Text:Use the References to access important values if needed for this question.
For the following reaction, 6.92 grams of carbon (graphite) are mixed with excess oxygen gas. The reaction yields 16.5 grams of carbon dioxide.
carbon (graphite) (s) + oxygen ( g ) -→ carbon dioxide ( g)
What is the theoretical yield of carbon dioxide ?
grams
What is the percent yield for this reaction ?
Submit Answer
Try Another Version
3 item attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co