Gallium has an atomic number of 31 and a mass number of 70. Which table shows the number of protons, neutrons, and electrons in a gallium ion with a +3 charge? * Particle Number Particle Number proton neutron proton neutron electron 39 31 31 39 electron 36 31 Option 1 Option 2 Particle Number proton Particle Number 39 neutron 31 proton 31 electron 39 neutron 39 electron 28
Gallium has an atomic number of 31 and a mass number of 70. Which table shows the number of protons, neutrons, and electrons in a gallium ion with a +3 charge? * Particle Number Particle Number proton neutron proton neutron electron 39 31 31 39 electron 36 31 Option 1 Option 2 Particle Number proton Particle Number 39 neutron 31 proton 31 electron 39 neutron 39 electron 28
Chapter3: Atoms And Elements
Section: Chapter Questions
Problem 19E: Give the atomic number (Z) and the mass number (A) for each of the following: a. a carbon atom with...
Related questions
Question
100%
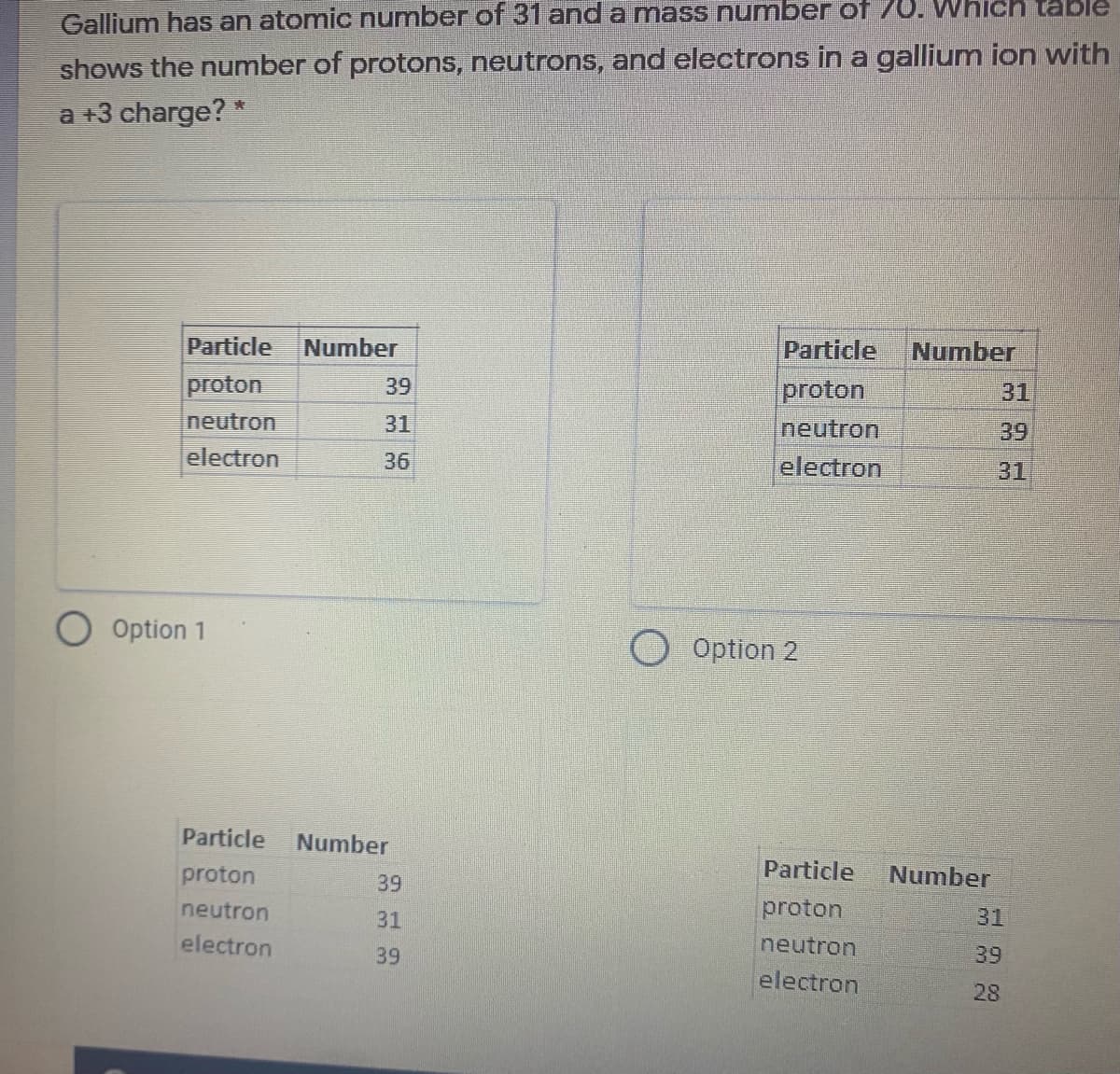
Transcribed Image Text:Gallium has an atomic number of 31 and a mass number of 70. Which table
shows the number of protons, neutrons, and electrons in a gallium ion with
a +3 charge? *
Particle
Number
Particle
Number
proton
proton
39
31
Ineutron
31
neutron
39
electron
36
electron
31
O Option 1
Option 2
Particle
Number
proton
Particle
Number
39
neutron
31
proton
31
electron
39
neutron
39
electron
28
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning