Read each description in the first column of the table below. If any chemical element with atomic number of 92 or less matches the description, check Yes and enter the chemical symbol of an element that matches. Otherwise check No in the second column. Does any element with ZS 92 match the description? If you checked yes, give the symbol of an element with z S 92 description that matches. An element in Period 3 and Group 2A. O Yes O No A metal in Group 7A. O Yes O No An alkali metal with a lower atomic O Yes O No number than calcium. A transition element in Period 6 O Yes O No
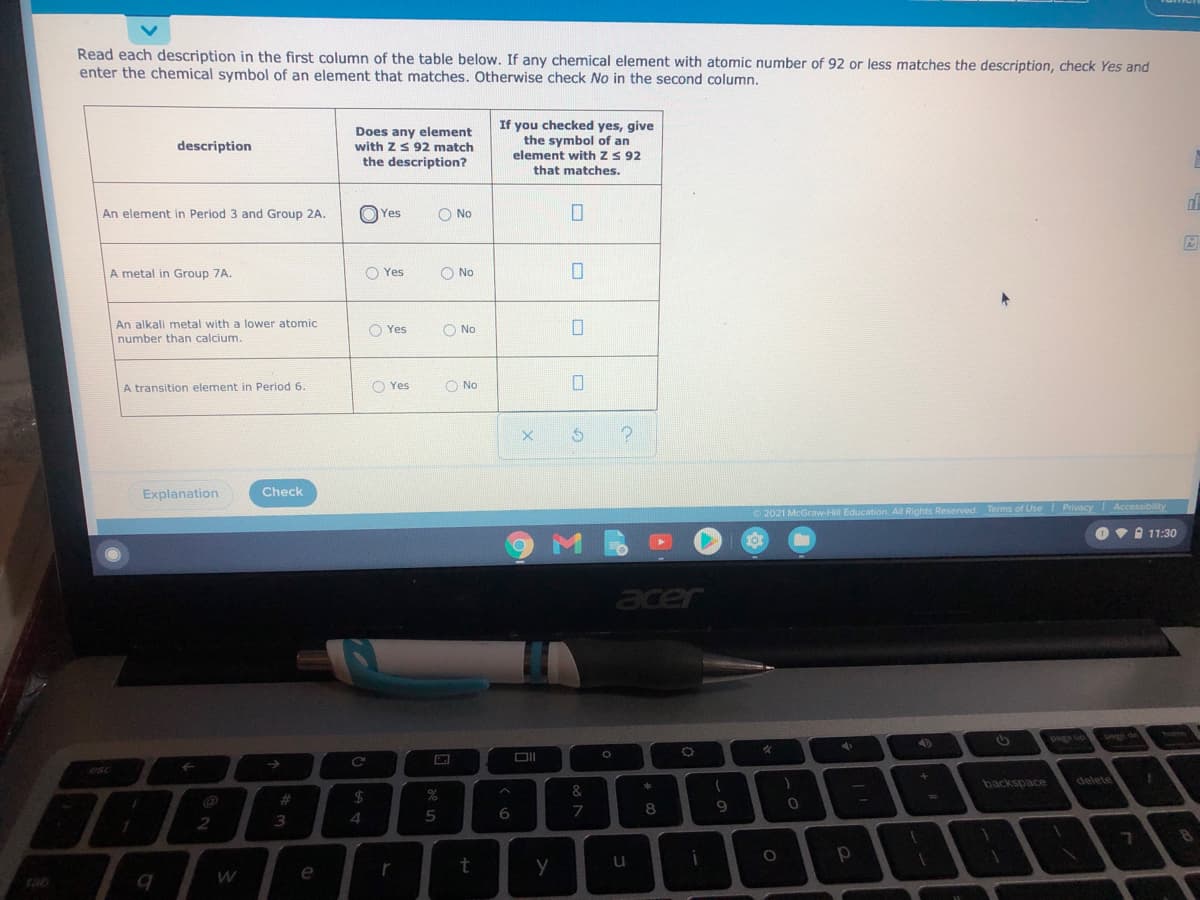
Atom Number (Z) should be equal or less than 92, which is of Uranium (U).
(1) An element in period 3 and group 2A -
In period 3 and group 2A , Magnesium is present with atomic number 12.
(2) A metal in group 7A -
In group 7A halogens are present. These are nonmetallic like - F, Cl, Br, I but At is metallic with atomic Number of 85.
(3) An alkali metal with with a lower atomic number than calcium -
Calcium (Ca) has atomic number of 20, while alkali metal is potassium (K) with atomic number of 19.
(4) A transition element in period 6 -
Period 6 contains transition elements of 5d - series, form Lanthanum (La-57) to Mercury (Hg-80). These are La, Hf Ta, W, Re, Os, Ir, Pt, Au and Hg.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps









