Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter14: Liquids And Solids
Section: Chapter Questions
Problem 18ALQ
Related questions
Question
100%
please solve for question 2 using the data above the reslt table.
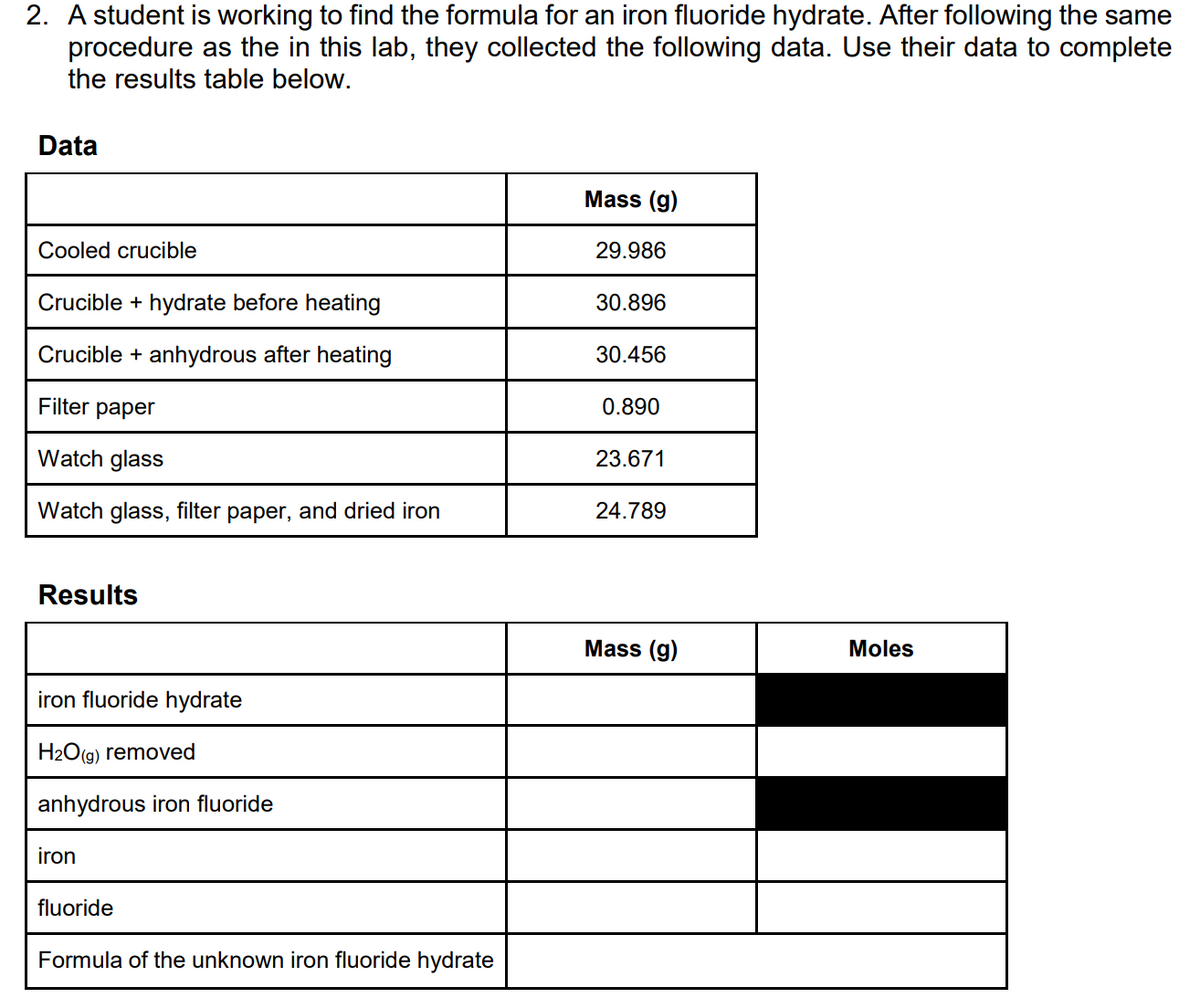
Transcribed Image Text:2. A student is working to find the formula for an iron fluoride hydrate. After following the same
procedure as the in this lab, they collected the following data. Use their data to complete
the results table below.
Data
Mass (g)
Cooled crucible
29.986
Crucible + hydrate before heating
30.896
Crucible + anhydrous after heating
30.456
Filter paper
0.890
Watch glass
23.671
Watch glass, filter paper, and dried iron
24.789
Results
Mass (g)
Moles
iron fluoride hydrate
H2O(g) removed
anhydrous iron fluoride
iron
fluoride
Formula of the unknown iron fluoride hydrate
Expert Solution
Step 1
Mass of hydrate = (30.896 - 29.986) g
= 0.91 g
Mass of anhydrous salt = (30.456-29.986) g
= 0.47 g
Mass of water removed = Mass of hydrate - Mass of anhydrous
= (0.91-0.47) g
= 0.44 g
Now,
No of moles = Mass / Molar Mass
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
I don't undertaand how you calculated the final ratio of H(2)O at the end of the explaination. How did you come to 6?
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning