Chapter8: Bonding: General Concepts
Section: Chapter Questions
Problem 152CP: Think of forming an ionic compound as three steps (this is a simplification, as with all models):...
Related questions
Question
100%
In the first picture, it should have the questions I answered and hopefully helps a bit on the question I have. I can’t find the electrons for the second picture in the blank yellow highlight.
Transcribed Image Text:ntify Bond Type
pols Add-ons Help
Last edit was 1 hour ago
kt
Arial
10.5
+
BIUA
川。 三三、三
2
3
4
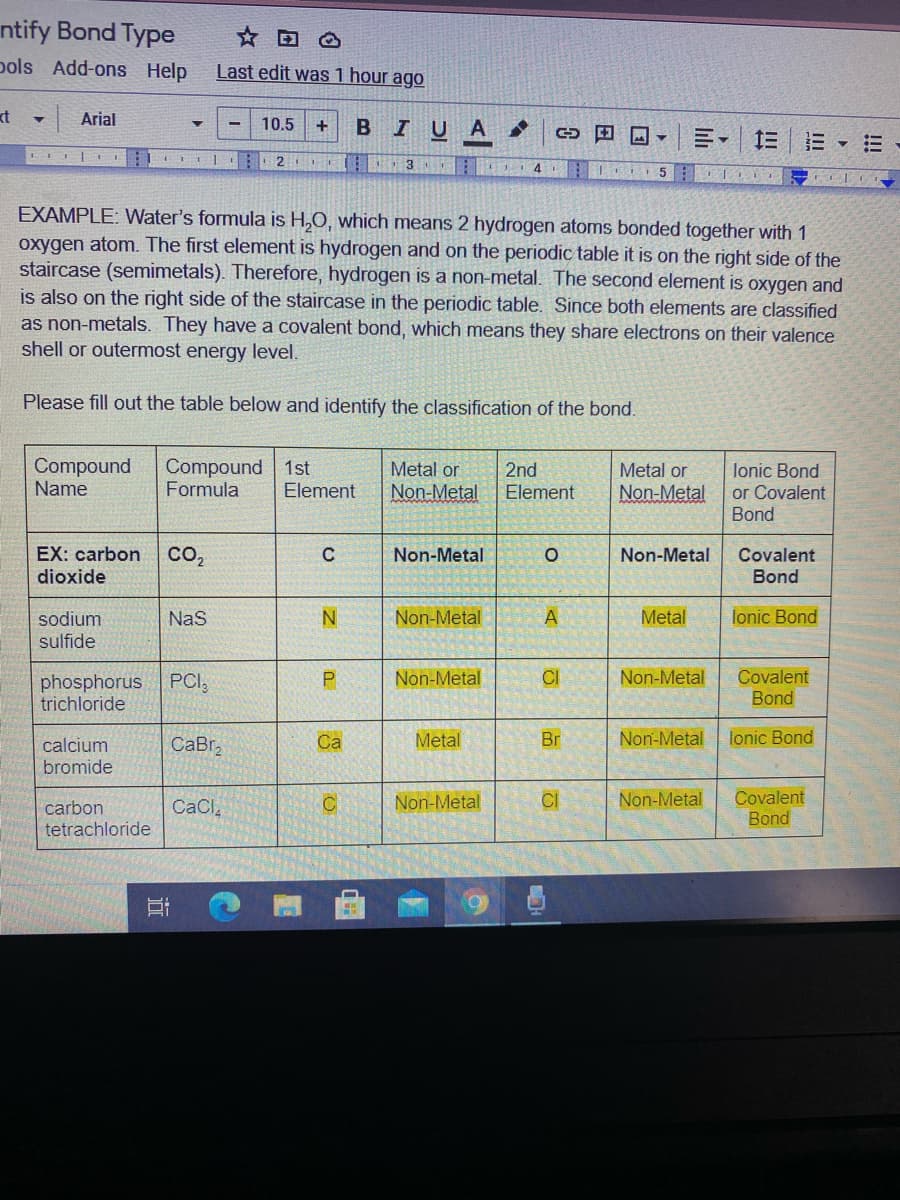
EXAMPLE: Water's formula is H,0, which means 2 hydrogen atoms bonded together with 1
oxygen atom. The first element is hydrogen and on the periodic table it is on the right side of the
staircase (semimetals). Therefore, hydrogen is a non-metal. The second element is oxygen and
is also on the right side of the staircase in the periodic table. Since both elements are classified
as non-metals. They have a covalent bond, which means they share electrons on their valence
shell or outermost energy level.
Please fill out the table below and identify the classification of the bond.
Compound
Name
Compound
Formula
1st
Metal or
Non-Metal
2nd
Element
Metal or
lonic Bond
Element
Non-Metal
or Covalent
Bond
EX: carbon
dioxide
Covalent
Bond
co,
Non-Metal
Non-Metal
Non-Metal
Metal
lonic Bond
sodium
sulfide
Nas
Covalent
Bond
phosphorus
trichloride
PCI,
Non-Metal
CI
Non-Metal
CaBr,
Ca
Metal
Br
Non-Metal
lonic Bond
calcium
bromide
Covalent
Bond
carbon
CaCl
Non-Metal
CI
Non-Metal
tetrachloride
近
Transcribed Image Text:E Bryan Rios Drafting Yo x
E Bryan Rios - Unit Hov x
Pt
ent/d/1_zfctlyURr8J7Lt5fbZ0zXQ-jg0QUXqMbPtqBkThpFM/edit
dentify Bond Type
Tools Add-ons Help
Last edit was 1 hour ago
l text
| Arial
10.5
BIUA
+
田回▼
三, 三|三▼
2
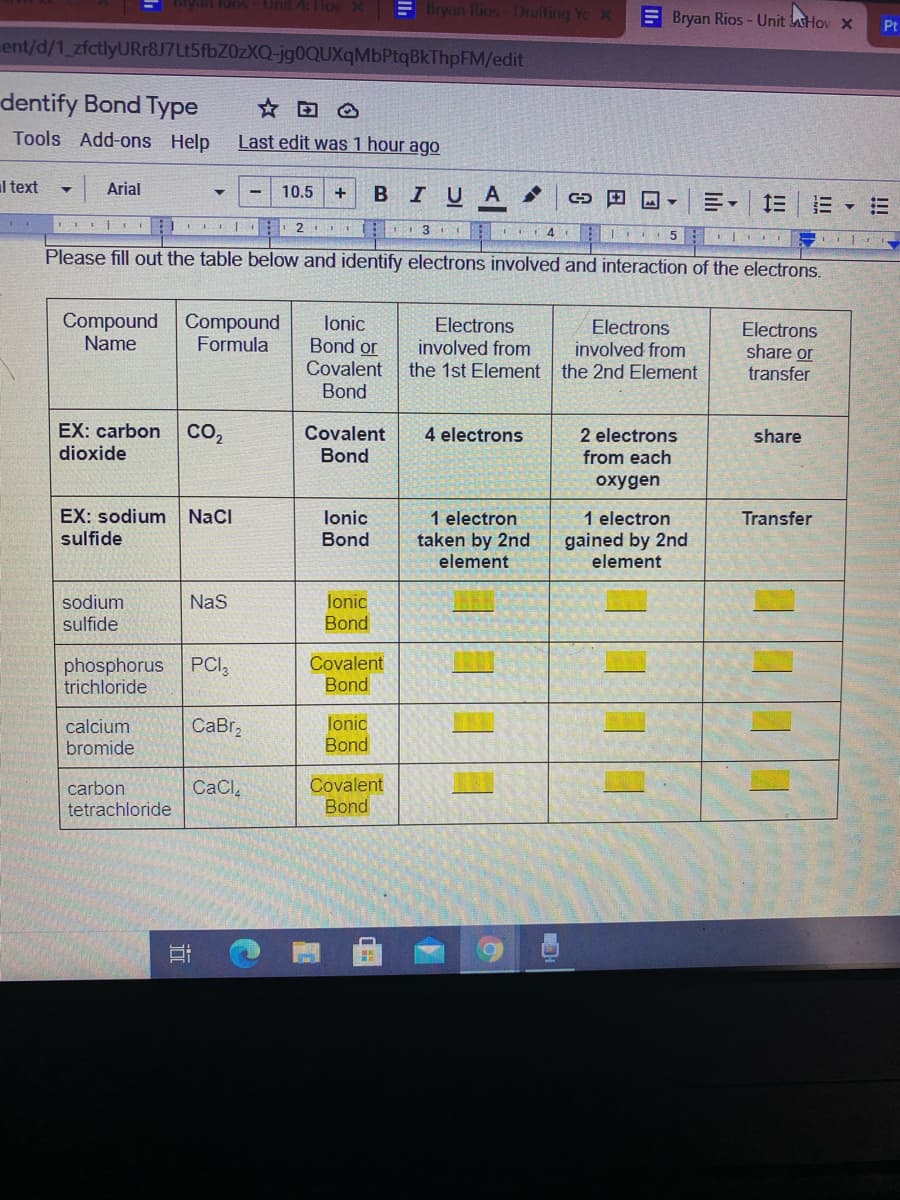
Please fill out the table below and identify electrons involved and interaction of the electrons.
Compound Compound
Name
lonic
Bond or
Covalent
Bond
Electrons
involved from
the 1st Element the 2nd Element
Electrons
involved from
Electrons
Formula
share or
transfer
EX: carbon
CO,
Covalent
4 electrons
2 electrons
share
dioxide
Bond
from each
oxygen
EX: sodium NaCI
sulfide
lonic
Bond
1 electron
1 electron
Transfer
taken by 2nd
element
gained by 2nd
element
sodium
sulfide
NaS
lonic
Bond
phosphorus
trichloride
PCI,
Covalent
Bond
CaBr,
lonic
Bond
calcium
bromide
Covalent
Bond
carbon
CaCl,
tetrachloride
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning