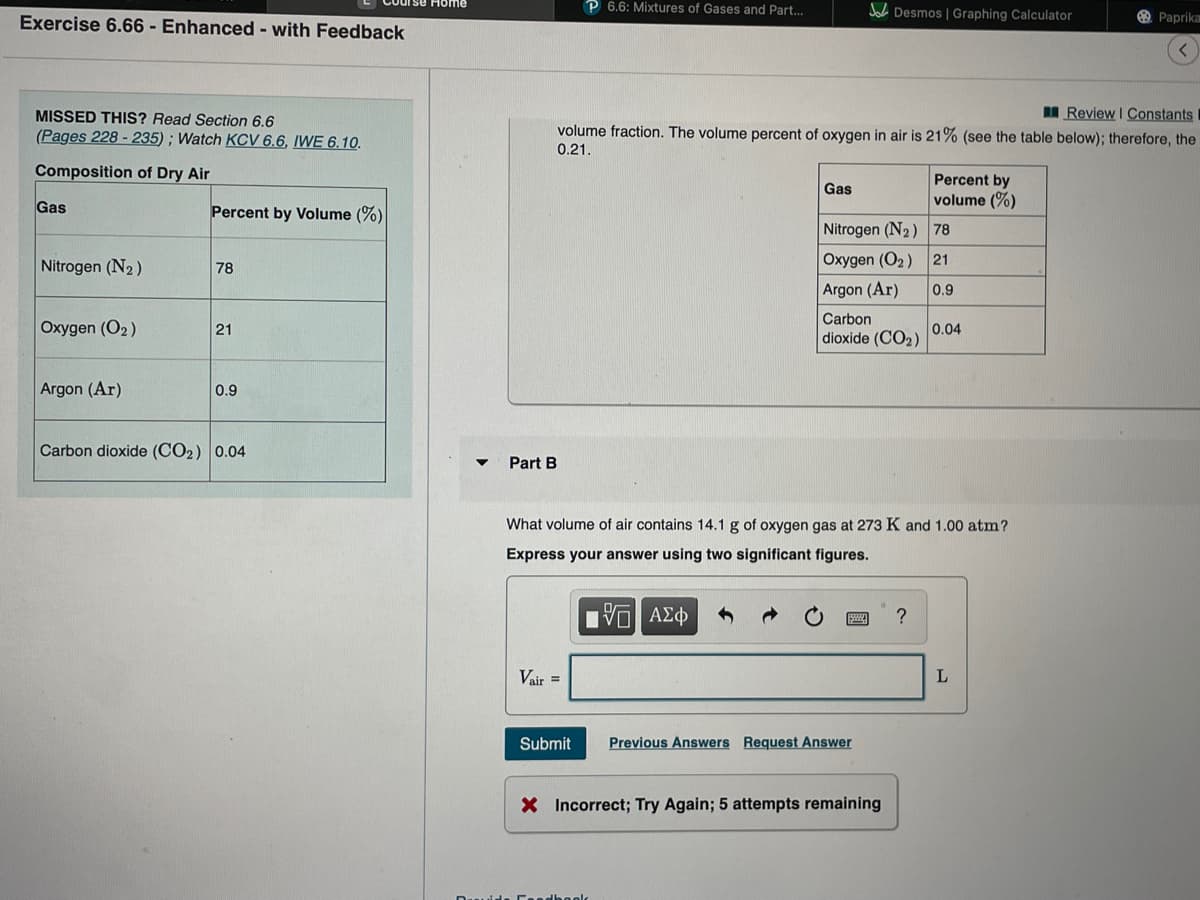
I Review I Constants SSED THIS? Read Section 6.6 ages 228 - 235) ; Watch KCV 6.6, IWE 6.10. volume fraction. The volume percent of oxygen in air is 21% (see the table below); therefore, the 0.21. mposition of Dry Air Percent by volume (%) Gas Percent by Volume (%) Nitrogen (N2) 78 trogen (N2 ) Oxygen (O2) 21 78 Argon (Ar) 0.9 kygen (O2) Carbon dioxide (CO2) 21 0.04 gon (Ar) 0.9 arbon dioxide (CO2) 0.04 Part B What volume of air contains 14.1 g of oxygen gas at 273 K and 1.00 atm? Express your answer using two significant figures. VO AEd ? Vair = Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining
I Review I Constants SSED THIS? Read Section 6.6 ages 228 - 235) ; Watch KCV 6.6, IWE 6.10. volume fraction. The volume percent of oxygen in air is 21% (see the table below); therefore, the 0.21. mposition of Dry Air Percent by volume (%) Gas Percent by Volume (%) Nitrogen (N2) 78 trogen (N2 ) Oxygen (O2) 21 78 Argon (Ar) 0.9 kygen (O2) Carbon dioxide (CO2) 21 0.04 gon (Ar) 0.9 arbon dioxide (CO2) 0.04 Part B What volume of air contains 14.1 g of oxygen gas at 273 K and 1.00 atm? Express your answer using two significant figures. VO AEd ? Vair = Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 79AP
Related questions
Question
Transcribed Image Text:rse Home
P 6.6: Mixtures of Gases and Part.
Jol Desmos | Graphing Calculator
Exercise 6.66 - Enhanced - with Feedback
Paprika
MISSED THIS? Read Section 6.6
(Pages 228 - 235) ; Watch KCV 6.6, IWE 6.10.
I Review I Constants
volume fraction. The volume percent of oxygen in air is 21% (see the table below); therefore, the
0.21.
Composition of Dry Air
Percent by
volume (%)
Gas
Gas
Percent by Volume (%)
Nitrogen (N2) 78
Nitrogen (N2)
Oxygen (O2 )
21
78
Argon (Ar)
0.9
Carbon
Oxygen (O2)
0.04
dioxide (CO2)
21
Argon (Ar)
0.9
Carbon dioxide (CO2) 0.04
Part
What volume of air contains 14.1 g of oxygen gas at 273 K and 1.00 atm?
Express your answer using two significant figures.
?
Vair =
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 5 attempts remaining
uide Ceedhoolc
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning