If the equation produces from data is y = 0.343x - 1.021 and the absorbance for the unknown is 0.386. What would be the concentration of the unknown. Give the answer with 3 decimal places.
If the equation produces from data is y = 0.343x - 1.021 and the absorbance for the unknown is 0.386. What would be the concentration of the unknown. Give the answer with 3 decimal places.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter4: Stoichiometry: Quantitative Information About Chemical Reactions
Section: Chapter Questions
Problem 77PS
Related questions
Question
Note: Answer is not 4.076
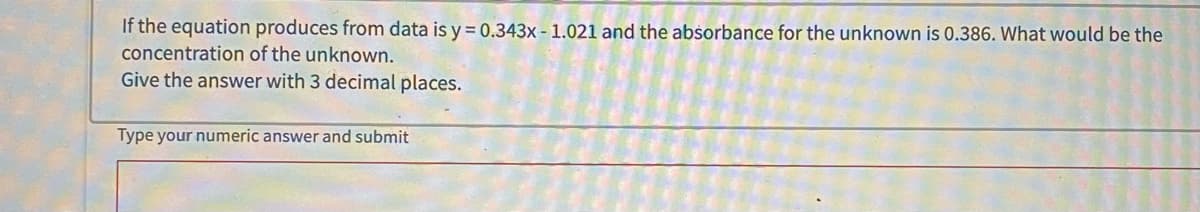
Transcribed Image Text:If the equation produces from data is y = 0.343x - 1.021 and the absorbance for the unknown is 0.386. What would be the
concentration of the unknown.
Give the answer with 3 decimal places.
Type your numeric answer and submit
Expert Solution
Step 1
Given data:
Equation obtained from graph, y = 0.343x – 1.021
Absorbance (A) = 0.386
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning