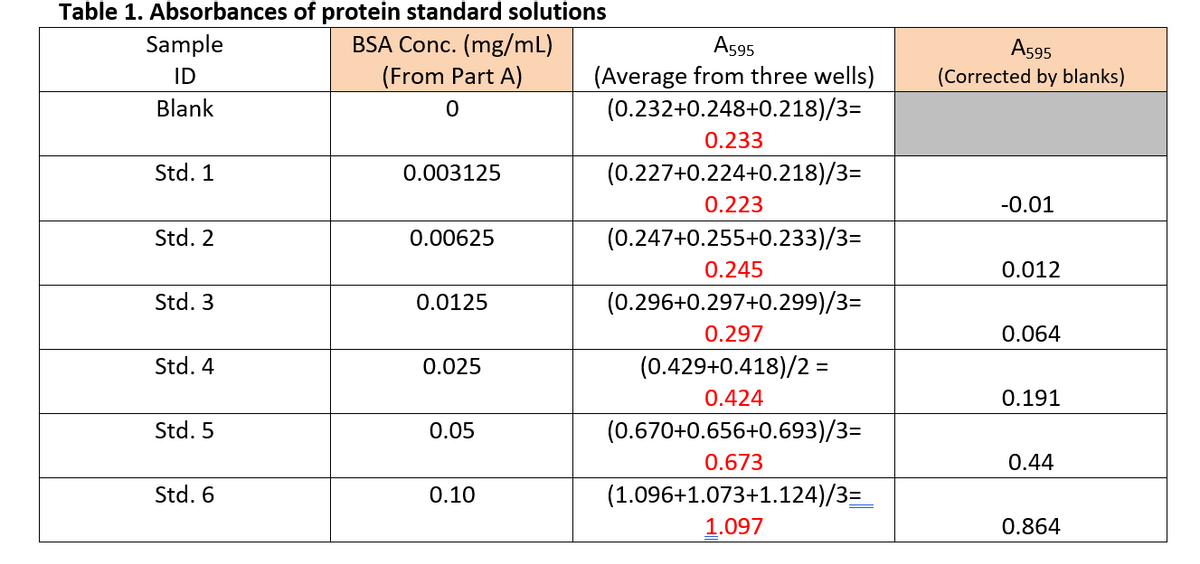
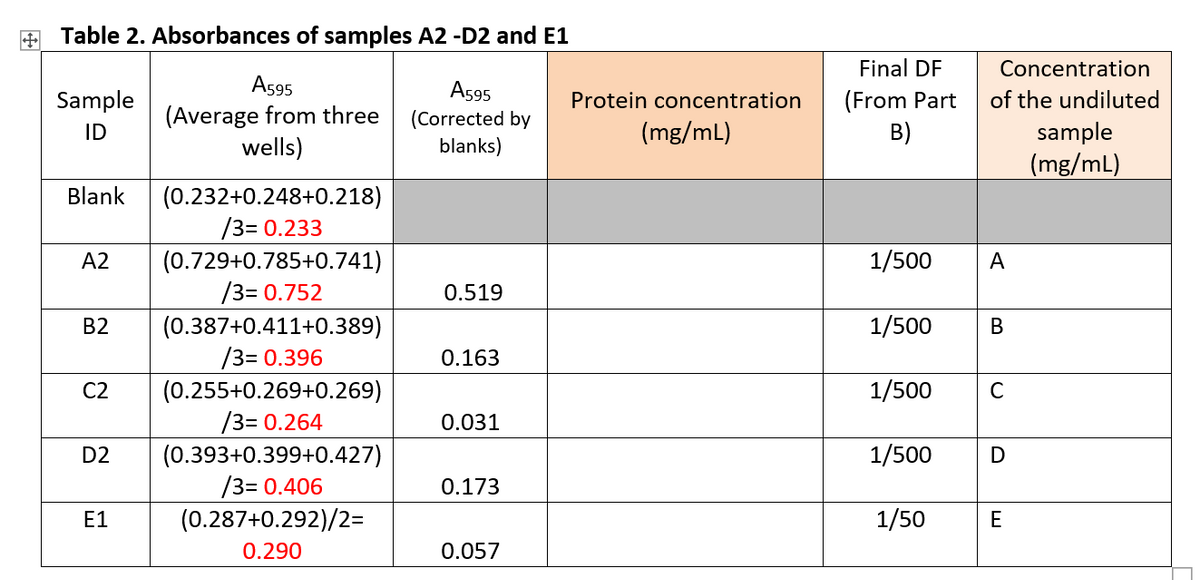
Please complete Table 2 and show the calculations for protein concentration and the concentration of the undiluted sample. a). Using Excel software, enter data from Table 1 to plot a standard curve (corrected average absorbance on Y-axis and protein concentrations on X-axis). The standard curve must contain correct labels on both axes. b). From the standard curve, obtain a best fit line and equation. c). Use the best fit equation and data from Table 2 to find the concentrations of proteins in sample A2-D2 and E1. [onto your plotted standard curve, indicate where A595 of A2-D2 and E1 are located] d). Calculate the concentrations of proteins in undiluted samples A to E. Thank you very much.
Please complete Table 2 and show the calculations for protein concentration and the concentration of the undiluted sample.
a). Using Excel software, enter data from Table 1 to plot a standard curve (corrected average absorbance on Y-axis and protein concentrations on X-axis). The standard curve must contain correct labels on both axes.
b). From the standard curve, obtain a best fit line and equation.
c). Use the best fit equation and data from Table 2 to find the concentrations of proteins in sample A2-D2 and E1. [onto your plotted standard curve, indicate where A595 of A2-D2 and E1 are located]
d). Calculate the concentrations of proteins in undiluted samples A to E.
Thank you very much.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 4 images








