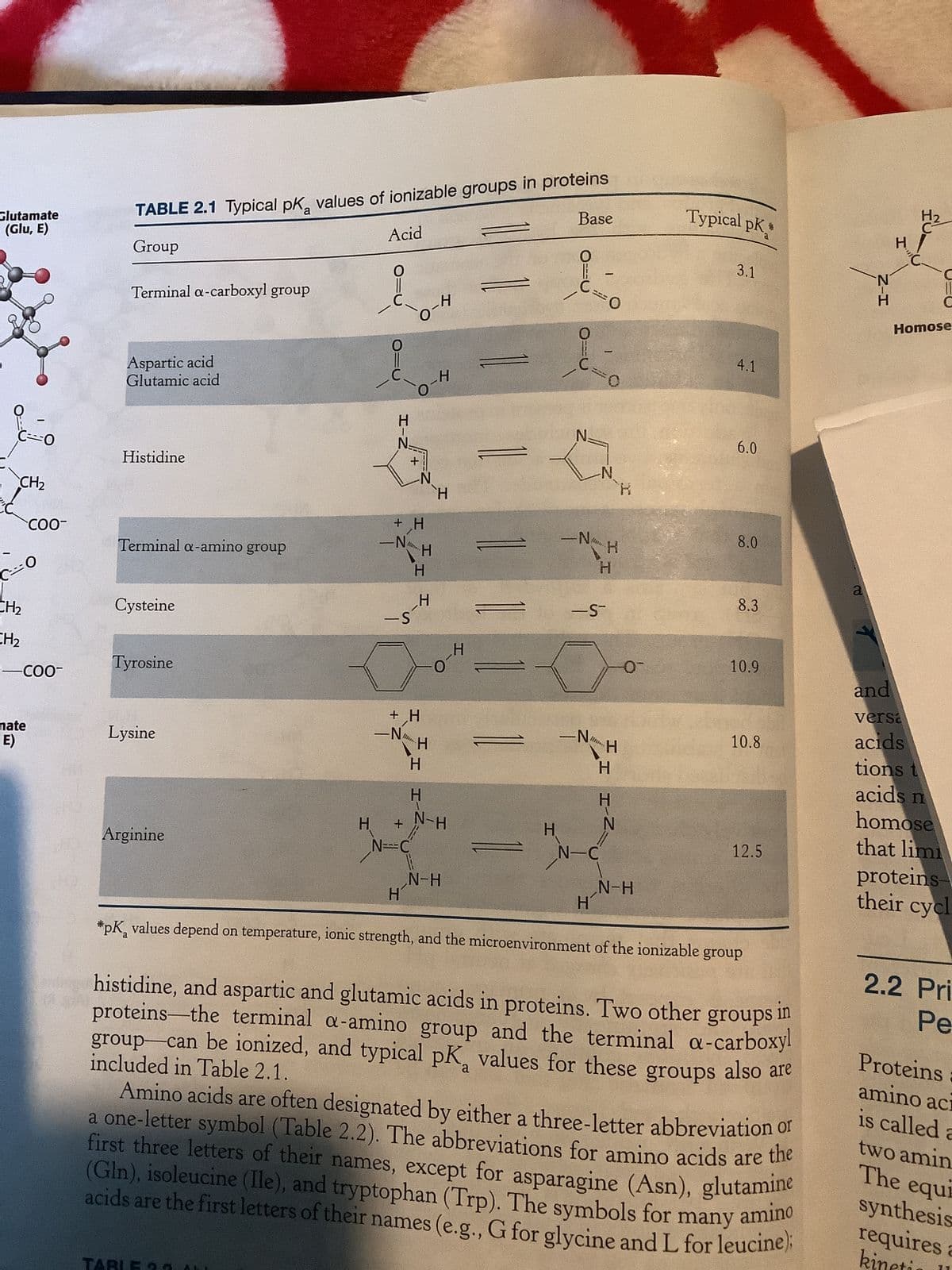
In each of the following cases, predict whether the pKa value of the first residue will be upshifted or downshifted relative to the typical pKa value (Table 2.1) based on the microenvironment. Briefly (in no more than 2-3 sentences) explain your reasoning. (a) A His residue adjacent to two Arg residues on the surface of a protein (b) A Cys residue adjacent to an Asp residue in the active site of an enzyme (c) A Lys residue buried in the hydrophobic core of a globular protein
In each of the following cases, predict whether the pKa value of the first residue will be upshifted or downshifted relative to the typical pKa value (Table 2.1) based on the microenvironment. Briefly (in
no more than 2-3 sentences) explain your reasoning.
(a) A His residue adjacent to two Arg residues on the surface of a protein
(b) A Cys residue adjacent to an Asp residue in the active site of an enzyme
(c) A Lys residue buried in the hydrophobic core of a globular protein
The pKa of a protein refers to the pH at which half of the protein's acidic residues (such as Asp or Glu) are protonated (have a hydrogen ion (H+) attached) and half are deprotonated (don't have a hydrogen ion attached). The pKa value is an important parameter for understanding the acid-base properties of a protein and how it interacts with its environment. It can affect a protein's stability, activity, and interactions with other molecules.
Step by step
Solved in 2 steps


