
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
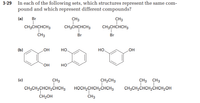
Transcribed Image Text:In each of the following sets, which structures represent the same com-
pound and which represent different compounds?
(a)
Br
CH3
CH3
CH3CHCHCH3
CH3CHCHCH3
CH3CHCHCH3
CH3
Br
Br
(b)
но.
но.
HO
HO
но
(c)
CH3
CH2CH3
CH3 CH3
CH3CH2CHCH2CHCH3
HOCH2CHCH2CHCH3
CH3CH2CHCH,CHCH2OH
CH2OH
CH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Similar questions
- 15) Which of the following structures is correct for 1,2-dibromo-2-methyl butane? CH₂-CH-CH₂Br Br CH₂CH₂-CH-CH₂ CH₂CH₂Br CH₂ A) C) CH₂CH3 BrCH₂-C-Br CH₂ B) D) BrCH₂-CH-CHCH₂ 1 Br CH₂arrow_forwardCompounds 1 and 2 were prepared, and the difference in their heats of combustion was found to be 17.2 kJ/mol (J. Am. Chem. Soc. 1961, 83, 606-614): H H 1 Shown below are the lowest-energy conformations of compounds 1 and 2. Identify which drawing matches which compound, and identify which compound has the larger heat of combustion. H H H H |||I 2 H 4 H H The first drawing is compound 1, and compound 1 has a larger heat of combustion because it is the less stable compound. O The first drawing is compound 2, and compound 2 has a larger heat of combustion because it is the less stable compound. The first drawing is compound 2, and compound 2 has a larger heat of combustion because it is the more stable compound. The first drawing is compound 1, and compound 1 has a larger heat of combustion because it is the more stable compound.arrow_forwardThe questions are on the imagearrow_forward
- Q22. (A) Write IUPAC names for the following compounds: A2.5 H₂C, CH₁ OH H,C-CH₂-CH-CH-CH₂-CH-CH₂-CH, L H₂C-CH-CH, CH, CH CH₂ T C C-CH-CH₂-CH₂ CH₁ CH₂ CH₂CH₂CHCHCH₂ OH O OHarrow_forwardGiven the structures of the following two tetramethylcyclohexanes: CH3 CH3 CH3 "CH3 CH3 CH3 A В (a) Draw the most stable chair conformation for both A and B. Which structure is lower in energy (most stable)? (b) Ring flip the above structures and draw the least stable chair conformation for both A and B. Which structure is highest in energy (least stable)?arrow_forward3-44 Draw the two chair conformations of each compound and label the substituents as axial and equatorial. In each case, deter- mine which conformation is more stable. (a) cis-1-ethyl-2-isopropylcyclohexane (c) cis-1-ethyl-3-methylcyclohexane (e) cis-1-ethyl-4-methylcyclohexane (d) (b) trans-1-ethyl-2-isopropylcyclohexane trans-1-ethyl-3-methylcyclohexane trans-1-ethyl-4-methylcyclohexane (f)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY