In Grignard reagent with bromobenzene and acetophenone, how would the reaction change if a student used ethanol as the solvent rather than diethyl ether? Dihydropyridir benzene would be the isolated product ethylbenzene would be the isolated product How can we c et would be an effective calcium channel blocker? the product would be unchanged, but the reaction rate would increase
In Grignard reagent with bromobenzene and acetophenone, how would the reaction change if a student used ethanol as the solvent rather than diethyl ether? Dihydropyridir benzene would be the isolated product ethylbenzene would be the isolated product How can we c et would be an effective calcium channel blocker? the product would be unchanged, but the reaction rate would increase
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter11: Ethers, Epoxides, And Sulfides
Section: Chapter Questions
Problem 11.18P
Related questions
Question
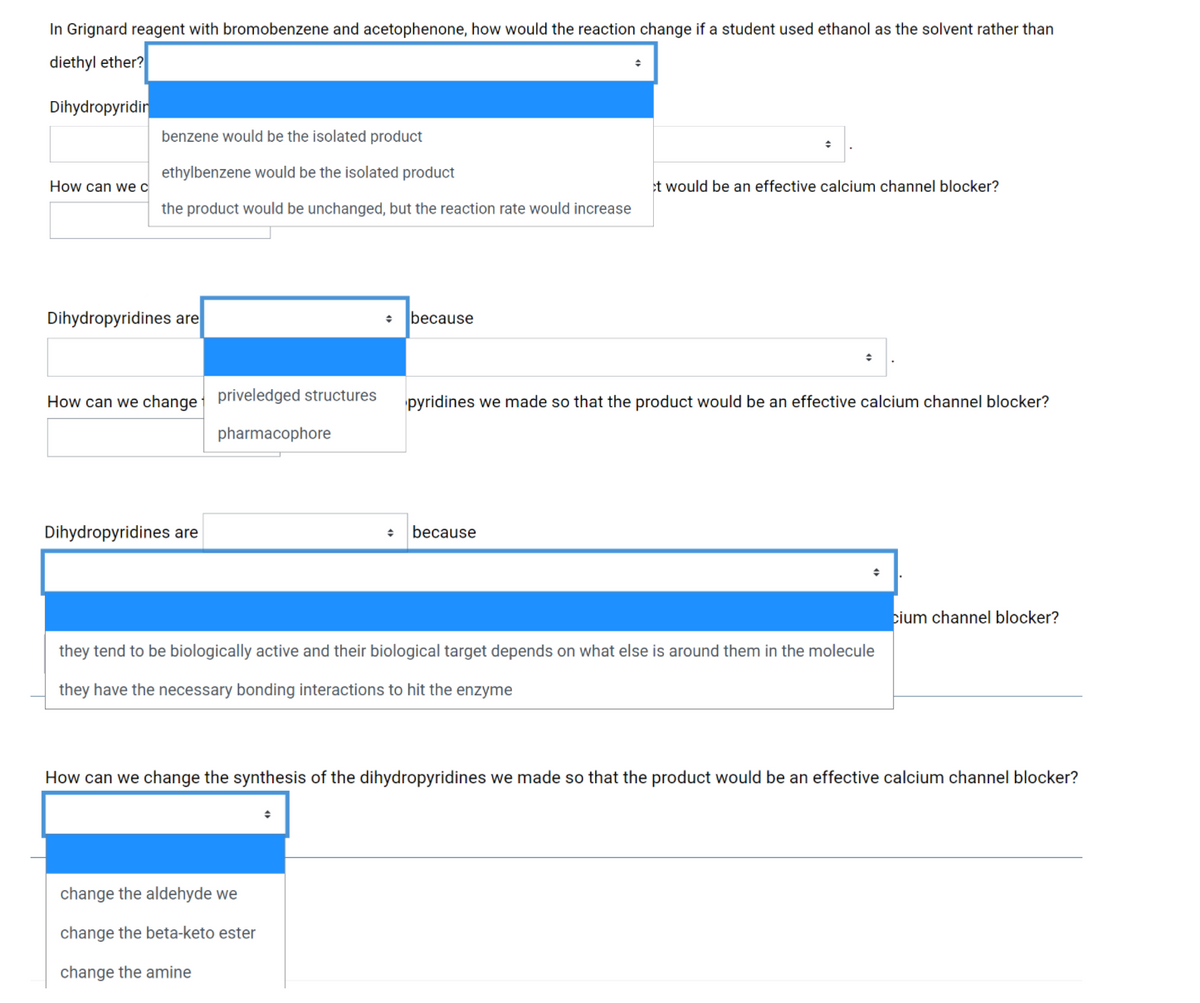
Transcribed Image Text:In Grignard reagent with bromobenzene and acetophenone, how would the reaction change if a student used ethanol as the solvent rather than
diethyl ether?
Dihydropyridin
benzene would be the isolated product
ethylbenzene would be the isolated product
How can we c
et would be an effective calcium channel blocker?
the product would be unchanged, but the reaction rate would increase
Dihydropyridines are
because
How can we change priveledged structures
pyridines we made so that the product would be an effective calcium channel blocker?
pharmacophore
Dihydropyridines are
because
cium channel blocker?
they tend to be biologically active and their biological target depends on what else is around them in the molecule
they have the necessary bonding interactions to hit the enzyme
How can we change the synthesis of the dihydropyridines we made so that the product would be an effective calcium channel blocker?
change the aldehyde we
change the beta-keto ester
change the amine
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole