In the process illustrated by the pV diagram in (Figure 1), the temperature of the ideal gas remains constant at 147 ∘C a) How many moles of gas are involved? Express your answer in moles to three significant figures. b) What volume does this gas occupy at a? Express your answer in cubic meters to three significant figures. c) How much work was done by or on the gas from a to b? Express your answer in joules to three significant figures. d) By how much did the internal energy of the gas change during this process? Express your answer in joules to three significant figures.
In the process illustrated by the pV diagram in (Figure 1), the temperature of the ideal gas remains constant at 147 ∘C a) How many moles of gas are involved? Express your answer in moles to three significant figures. b) What volume does this gas occupy at a? Express your answer in cubic meters to three significant figures. c) How much work was done by or on the gas from a to b? Express your answer in joules to three significant figures. d) By how much did the internal energy of the gas change during this process? Express your answer in joules to three significant figures.
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter4: Introduction To Gases
Section: Chapter Questions
Problem 65E: If 1.62 m3 of air at 120C and 738 torr is compressed into a 0.140 m3 tank, and the temperature is...
Related questions
Question
In the process illustrated by the pV diagram in (Figure 1), the temperature of the ideal gas remains constant at 147 ∘C
a) How many moles of gas are involved? Express your answer in moles to three significant figures.
b) What volume does this gas occupy at a? Express your answer in cubic meters to three significant figures.
c) How much work was done by or on the gas from a to b? Express your answer in joules to three significant figures.
d) By how much did the internal energy of the gas change during this process? Express your answer in joules to three significant figures.
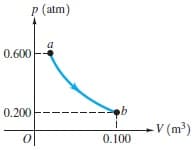
Transcribed Image Text:P (atm)
a
0.600
0.200
V (m³)
0.100
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax