Metric Measurement and Scientific Notation LAB 1 Going up the ladder, move the decimal point to the LEFT Going down the ladder, move the decimal point to the RIGHT kilo hecto deka BASE UNIT (meter, liter, gram) deci centi milli ©Hayden-McNeil, LLC Try converting nanograms into centigrams using Table 1. Note that the prefix for nano is 10-9 and centi is 10-2. Nano is smaller than centi so the decimal will be moved to the left. The difference between the exponential values is 7. So the decimal will be moved 7 places to the left. 1 nanometer = 0.0000001 centimeters. Complete the following metric conversions. m 16. 4 dm 13. 16 nm mm mg 17. 1235 kg kg 14. 245 g dm 18. 98 mm kL %3D 15. 23 mL 3.
Metric Measurement and Scientific Notation LAB 1 Going up the ladder, move the decimal point to the LEFT Going down the ladder, move the decimal point to the RIGHT kilo hecto deka BASE UNIT (meter, liter, gram) deci centi milli ©Hayden-McNeil, LLC Try converting nanograms into centigrams using Table 1. Note that the prefix for nano is 10-9 and centi is 10-2. Nano is smaller than centi so the decimal will be moved to the left. The difference between the exponential values is 7. So the decimal will be moved 7 places to the left. 1 nanometer = 0.0000001 centimeters. Complete the following metric conversions. m 16. 4 dm 13. 16 nm mm mg 17. 1235 kg kg 14. 245 g dm 18. 98 mm kL %3D 15. 23 mL 3.
Chapter1: Matter, Measurements, And Calculations
Section: Chapter Questions
Problem 1.33E
Related questions
Question
How do you answer 13, 14, and 15?
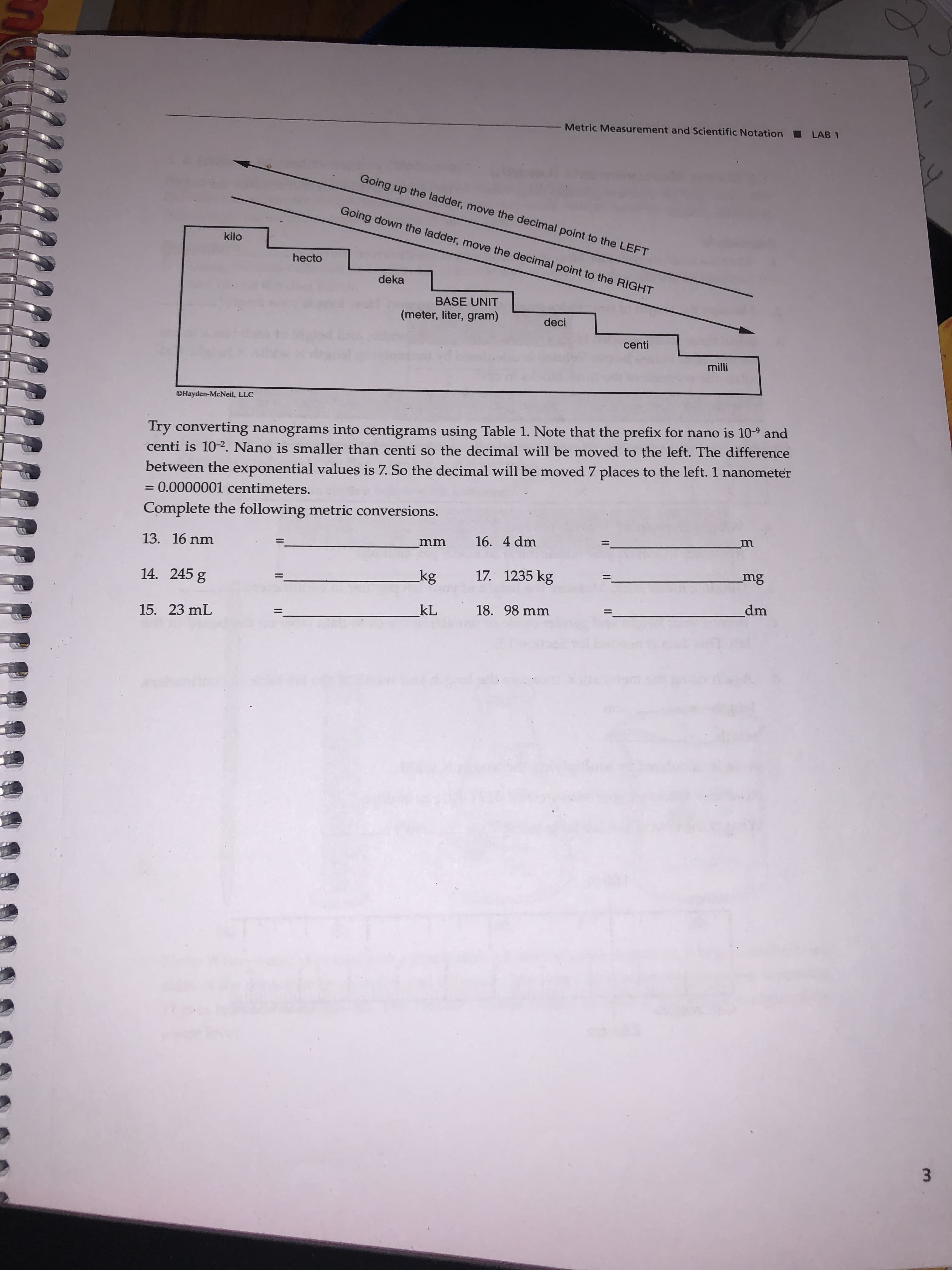
Transcribed Image Text:Metric Measurement and Scientific Notation
LAB 1
Going up the ladder, move the decimal point to the LEFT
Going down the ladder, move the decimal point to the RIGHT
kilo
hecto
deka
BASE UNIT
(meter, liter, gram)
deci
centi
milli
©Hayden-McNeil, LLC
Try converting nanograms into centigrams using Table 1. Note that the prefix for nano is 10-9 and
centi is 10-2. Nano is smaller than centi so the decimal will be moved to the left. The difference
between the exponential values is 7. So the decimal will be moved 7 places to the left. 1 nanometer
= 0.0000001 centimeters.
Complete the following metric conversions.
m
16. 4 dm
13. 16 nm
mm
mg
17. 1235 kg
kg
14. 245 g
dm
18. 98 mm
kL
%3D
15. 23 mL
3.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning