Mixing 50.0 mL of 0.200 M Na2HPO4 with 50.0 mL of 0.120 M HCI results in a buffer solution composed of: H3PO4 and NaH,PO4 NazHPO4 and NagPO4 NaH,PO4 and NazHPO4 NaH2PO4 and Na3PO4
Mixing 50.0 mL of 0.200 M Na2HPO4 with 50.0 mL of 0.120 M HCI results in a buffer solution composed of: H3PO4 and NaH,PO4 NazHPO4 and NagPO4 NaH,PO4 and NazHPO4 NaH2PO4 and Na3PO4
Biology (MindTap Course List)
11th Edition
ISBN:9781337392938
Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Chapter2: Atoms And Molecules: The Chemical Basis Of Life
Section2.6: Acids, Bases, And Salts
Problem 1C
Related questions
Question
5
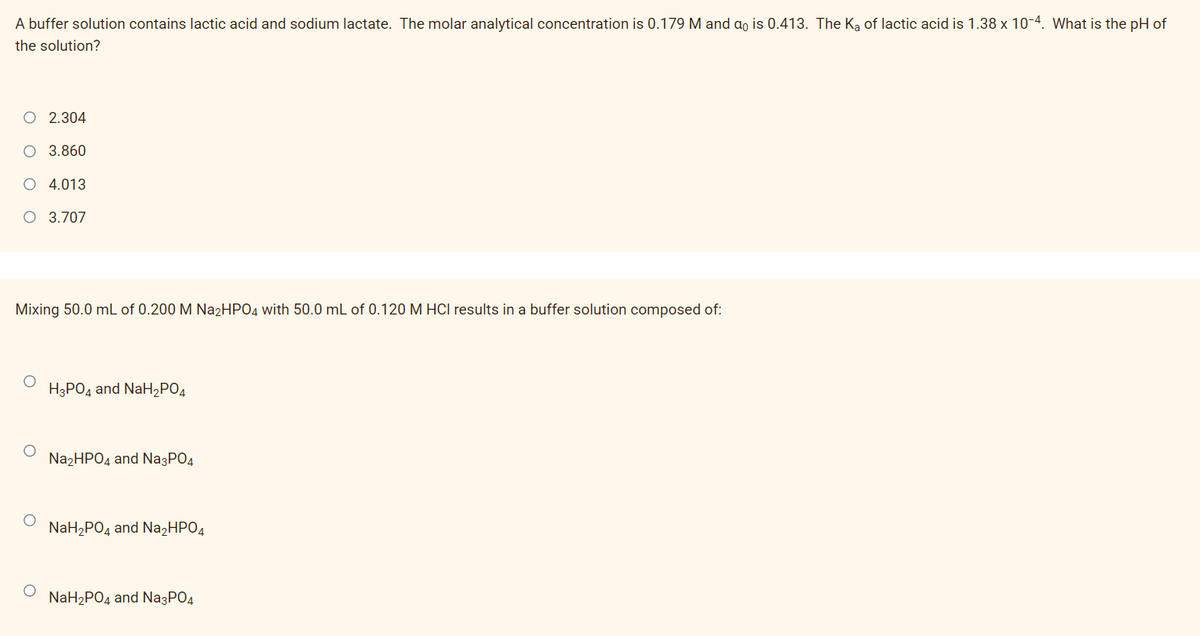
Transcribed Image Text:A buffer solution contains lactic acid and sodium lactate. The molar analytical concentration is 0.179 M and a, is 0.413. The Ka of lactic acid is 1.38 x 10-4. What is the pH of
the solution?
O 2.304
O 3.860
4.013
O 3.707
Mixing 50.0 mL of 0.200 M Na2HPO4 with 50.0 mL of 0.120 M HCI results in a buffer solution composed of:
НЭРОД and NaHzРОд
N22HPO4 and Na3PO4
NaH,PO4 and Na,HPO4
NaH2PO4 and Na3PO4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Biology (MindTap Course List)
Biology
ISBN:
9781337392938
Author:
Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:
Cengage Learning



Biology (MindTap Course List)
Biology
ISBN:
9781337392938
Author:
Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:
Cengage Learning



Basic Clinical Lab Competencies for Respiratory C…
Nursing
ISBN:
9781285244662
Author:
White
Publisher:
Cengage


Essentials of Pharmacology for Health Professions
Nursing
ISBN:
9781305441620
Author:
WOODROW
Publisher:
Cengage