Morality of HCI: 12M (mol/L) Number of mole of HCI used: = 15mL x 1L/1000mL x 12mol/1L= 0.18 mole HC1 wwwm Molecular Weight of (CH3)3COH: 74.12 g/Mol Number of mole of (CH3)3COH(tert-butanol) used: = 4.0g x 1mol/ 74.12g = 0.0539 mole (CH3)3COH Based on the above calculations, we know the limiting reagent is tert-butanol Based on our balanced equations for the reaction, we have a ration of 1:1 mole to find our theoretical yield for our product: (CH3)3COH(aq) + HCI (aq) — (CH3)3CC1 (aq) + H20 (1) Molecular weight of (CH3)3CCI: 92.75% g/mol Theoretical Yield of (CH3)3CCI (tert-butyl chloride): = 0.0539 mol (CH3)3COH x 1 mole (CH3)3CC1/ 1mole (CH3)3COH x 92.75g(CH3)3CCI/1mol of (CH3)3CCI=4.99g| Percent Yield: Percent Yield = (experimental value/ theoretical yield) x 100% = (1.89g/4.99g) x 100% = 37.88%
Catalysis and Enzymatic Reactions
Catalysis is the kind of chemical reaction in which the rate (speed) of a reaction is enhanced by the catalyst which is not consumed during the process of reaction and afterward it is removed when the catalyst is not used to make up the impurity in the product. The enzymatic reaction is the reaction that is catalyzed via enzymes.
Lock And Key Model
The lock-and-key model is used to describe the catalytic enzyme activity, based on the interaction between enzyme and substrate. This model considers the lock as an enzyme and the key as a substrate to explain this model. The concept of how a unique distinct key only can have the access to open a particular lock resembles how the specific substrate can only fit into the particular active site of the enzyme. This is significant in understanding the intermolecular interaction between proteins and plays a vital role in drug interaction.
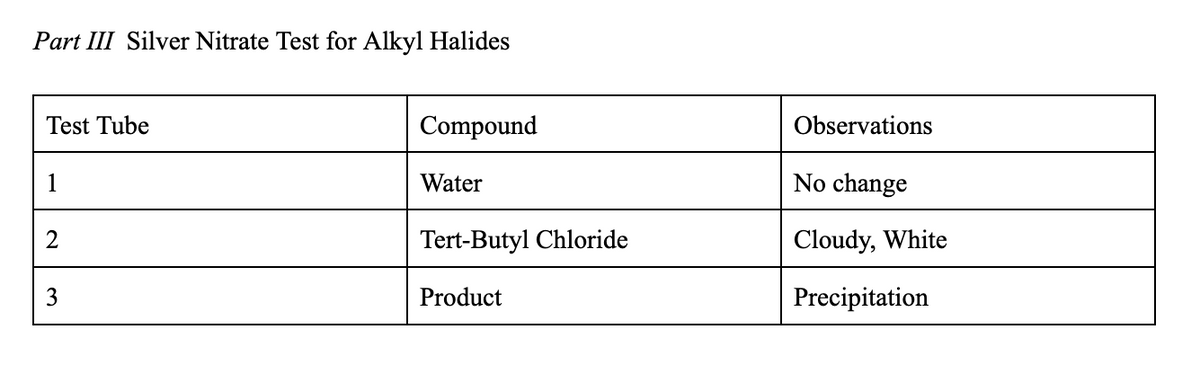
In the reaction observed in part III, where is the slow step? do you expect the reaction in part III to be faster for tert-butyl chloride or 2-chloropropane and why?
Part III Silver Nitrate Test for Tertiary
- Set up the reaction.
- Obtain three 13 100 test tubes and label 13.
- Pipet 10 drops of distilled water into Test Tube 1.
- Pipet 10 drops of tert-butyl chloride into Test Tube 2
- Pipet 10 drops of your product into Test Tube 3.
- Add approximately 1 mL of the 1% ethanolic silver nitrate solution to each test tube. Swirl each test tube to mix the contents. The appearance of a white precipitate indicates the presence of a 3° halide. Record your observations in the data table. Note: Avoid getting the AgNO3 solution on your skin.
- At the end of the experiment, discard the solutions as directed by your instructor.
IMAGES PROVIDED

Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images




