Observe the graph below. According to the chart shown above, if the saturated solution of glucose at 80°C was suddenly cooled to 40°C, what would happen? Solubility of Glucose in Water 600 500 400 300 200 100 20 40 60 80 100 Temperature (Celsius) An additional 300 grams of glucose could dissolve. The solution would spontaneously cool to 200°C. The solution becomes supersaturated. Nothing special would happen; the increased temperature just made the dissolving process quicker. Dissolved Mass (grams)
Observe the graph below. According to the chart shown above, if the saturated solution of glucose at 80°C was suddenly cooled to 40°C, what would happen? Solubility of Glucose in Water 600 500 400 300 200 100 20 40 60 80 100 Temperature (Celsius) An additional 300 grams of glucose could dissolve. The solution would spontaneously cool to 200°C. The solution becomes supersaturated. Nothing special would happen; the increased temperature just made the dissolving process quicker. Dissolved Mass (grams)
Chapter7: Solutions And Colloids
Section: Chapter Questions
Problem 7.5E
Related questions
Question
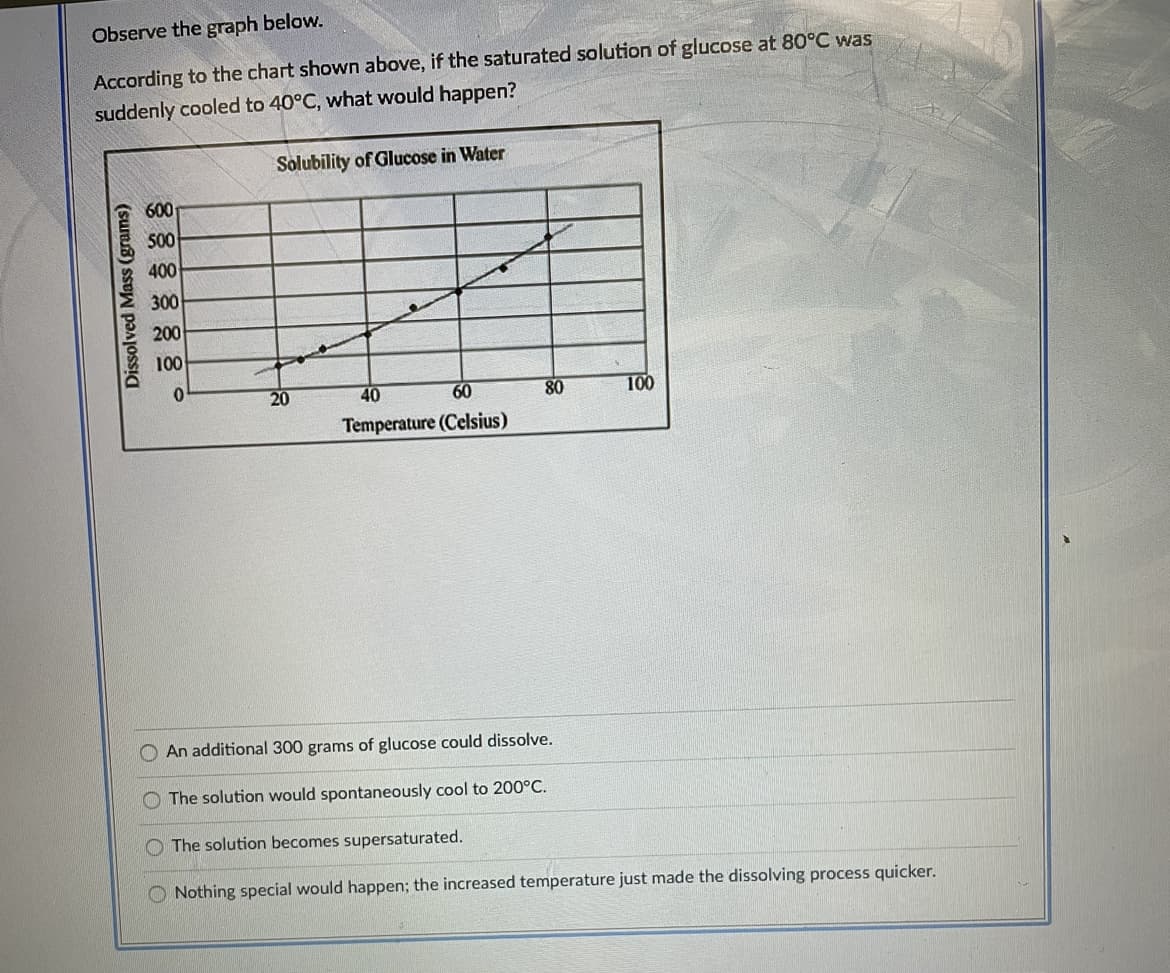
Transcribed Image Text:Observe the graph below.
According to the chart shown above, if the saturated solution of glucose at 80°C was
suddenly cooled to 40°C, what would happen?
Solubility of Glucose in Water
600
S00
400
300
200
100
20
40
80
100
Temperature (Celsius)
An additional 300 grams of glucose could dissolve.
O The solution would spontaneously cool to 200°C.
O The solution becomes supersaturated.
O Nothing special would happen; the increased temperature just made the dissolving process quicker.
Dissolved Mass (grams)

Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning