of the system, decrease S, or leave S unchanged. If you don't have enough information to decide, check t Note for advanced students: you may assume ideal gas and ideal solution behaviour. System Change AS O aS < o A mixture of helium (He) gas and O AS = 0 An additional 2.0 L of pure Ar gas is added to the mixture, with the pressure kept constant at 5 atm. argon (Ar) gas at 5 atm and O As > 0 -14°C. not enough information AS < 0 O AS = 0 A solution made of sodium iodide 50. mL of pure water is added to (Nal) in water, at 92°C. O As > 0 the solution. not enough information AS < 0 A 0.35 M solution of sucrose in The solution is put into a semipermeable bag immersed in the O AS = 0 water, and a beaker of pure water, water, and 50. mL of pure water O As > 0 both at 37.°C. flows through the bag into the sucrose solution. not enough information
of the system, decrease S, or leave S unchanged. If you don't have enough information to decide, check t Note for advanced students: you may assume ideal gas and ideal solution behaviour. System Change AS O aS < o A mixture of helium (He) gas and O AS = 0 An additional 2.0 L of pure Ar gas is added to the mixture, with the pressure kept constant at 5 atm. argon (Ar) gas at 5 atm and O As > 0 -14°C. not enough information AS < 0 O AS = 0 A solution made of sodium iodide 50. mL of pure water is added to (Nal) in water, at 92°C. O As > 0 the solution. not enough information AS < 0 A 0.35 M solution of sucrose in The solution is put into a semipermeable bag immersed in the O AS = 0 water, and a beaker of pure water, water, and 50. mL of pure water O As > 0 both at 37.°C. flows through the bag into the sucrose solution. not enough information
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter4: Gibbs Energy And Chemical Potential
Section: Chapter Questions
Problem 4.1E: List the sets of conditions that allow dS, dU, and dH of a process in a system act as a spontaneity...
Related questions
Question
100%
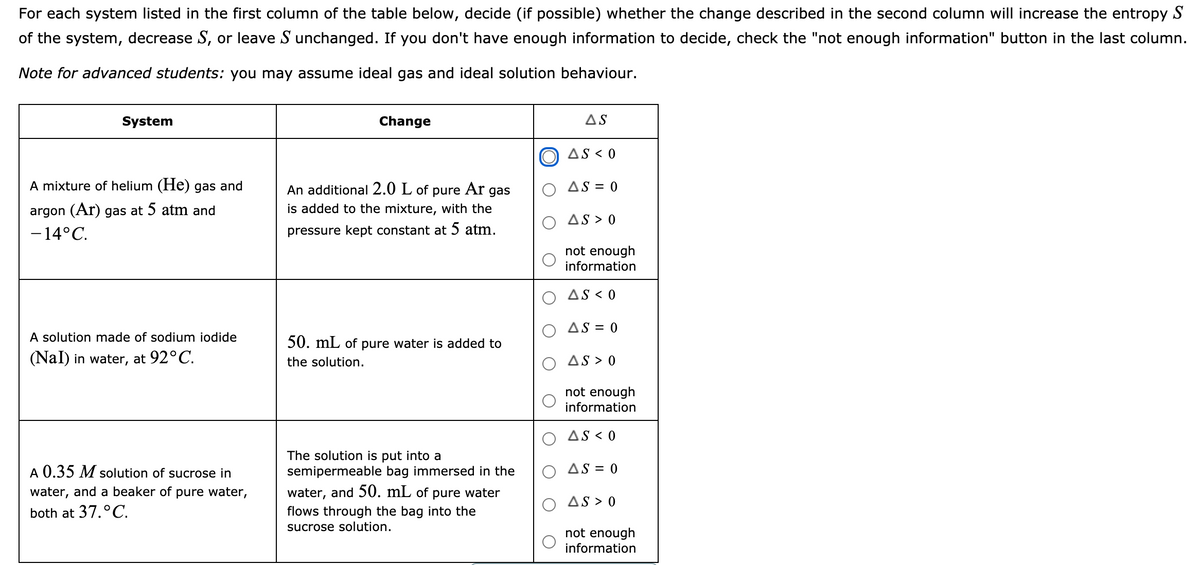
Transcribed Image Text:For each system listed in the first column of the table below, decide (if possible) whether the change described in the second column will increase the entropy S
of the system, decrease S, or leave S unchanged. If you don't have enough information to decide, check the "not enough information" button in the last column.
Note for advanced students: you may assume ideal gas and ideal solution behaviour.
System
Change
AS
AS < 0
A mixture of helium (He) gas and
Ar
AS = 0
An additional 2.0 L of
is added to the mixture, with the
pure
gas
argon (Ar) gas at 5 atm and
AS > 0
-14°C.
pressure kept constant at 5 atm.
not enough
information
AS < 0
AS = 0
A solution made of sodium iodide
50. mL of pure water is added to
(Nal) in water, at 92°C.
the solution.
AS > 0
not enough
information
AS < 0
The solution is put into a
semipermeable bag immersed in the
A 0.35 M solution of sucrose in
O AS = 0
water, and a beaker of pure water,
water, and 50. mL of pure water
AS > 0
both at 37.°C.
flows through the bag into the
sucrose solution.
not enough
information
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,