Phosgene (carbonyl chloride), COCL2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. Phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: CO(g) + Cl2 (g) = COC22 (g) Carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 473 °C . At equilibrium, the concentrations were measured and the following results obtained: Partial Pressure Gas (atm) CO 0.810 Cl2 1.19 COCI2 0.280 What is the equilibrium constant, Kp, of this reaction?
Phosgene (carbonyl chloride), COCL2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. Phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: CO(g) + Cl2 (g) = COC22 (g) Carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 473 °C . At equilibrium, the concentrations were measured and the following results obtained: Partial Pressure Gas (atm) CO 0.810 Cl2 1.19 COCI2 0.280 What is the equilibrium constant, Kp, of this reaction?
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter12: Gaseous Chemical Equilibrium
Section: Chapter Questions
Problem 62QAP: Mustard gas, used in chemical warfare in World War I, has been found to be an effective agent in the...
Related questions
Question
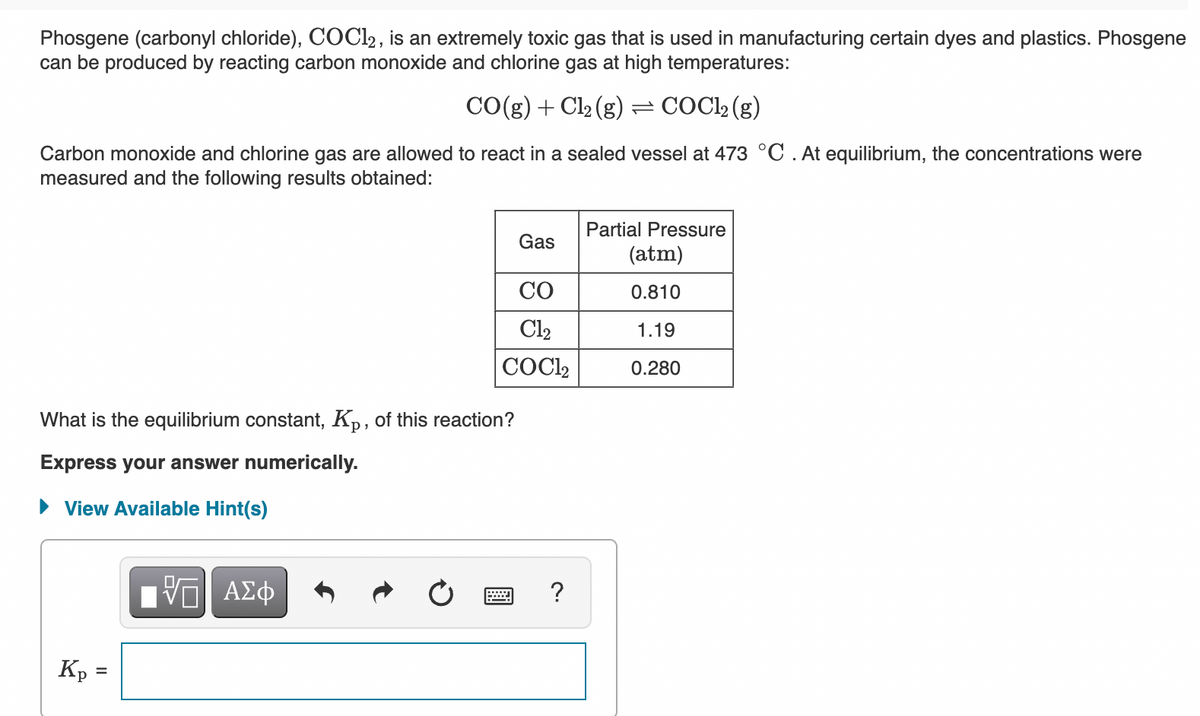
Transcribed Image Text:Phosgene (carbonyl chloride), COCL2 , is an extremely toxic gas that is used in manufacturing certain dyes and plastics. Phosgene
can be produced by reacting carbon monoxide and chlorine gas at high temperatures:
CO(g) + Cl2 (g) = COC2 (g)
Carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 473 °C.At equilibrium, the concentrations were
measured and the following results obtained:
Partial Pressure
Gas
(atm)
CO
0.810
Cl2
1.19
COC2
0.280
What is the equilibrium constant, Kp, of this reaction?
Express your answer numerically.
View Available Hint(s)
ΑΣφ
?
Kp
II
Expert Solution
Step 1
Kp is the ratio of the equilibrium partial pressure of products over equilibrium partial pressure of reactants each raised to the power of their stoichiometric coefficients.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
