QUESTION 1 a) 13 moles of monatomic ideal gas undergoes a thermodynamic process from point a to point c, as shown by the PV diagram in Figure 1. 13 mol gas ideal monatom mengalami proses termodinamik dari titik a ke titik c, seperti yang ditunjukkan oleh rajah PV dalam Rajah 1. P (Pa) 4.0 x 10 2.0 X 10 V (m) 0 0.001 0.002 0.003 0.004 Figure 1/ Rajah 1 Determine the work done ON the gas from point a to point c. Find the temperature of the gas at point b. ASPO504 SET ३
QUESTION 1 a) 13 moles of monatomic ideal gas undergoes a thermodynamic process from point a to point c, as shown by the PV diagram in Figure 1. 13 mol gas ideal monatom mengalami proses termodinamik dari titik a ke titik c, seperti yang ditunjukkan oleh rajah PV dalam Rajah 1. P (Pa) 4.0 x 10 2.0 X 10 V (m) 0 0.001 0.002 0.003 0.004 Figure 1/ Rajah 1 Determine the work done ON the gas from point a to point c. Find the temperature of the gas at point b. ASPO504 SET ३
Physics for Scientists and Engineers, Technology Update (No access codes included)
9th Edition
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter21: The Kinetic Theory Of Gases
Section: Chapter Questions
Problem 21.55AP: Model air as a diatomic ideal gas with M = 28.9 g/mol. A cylinder with a piston contains 1.20 kg of...
Related questions
Question
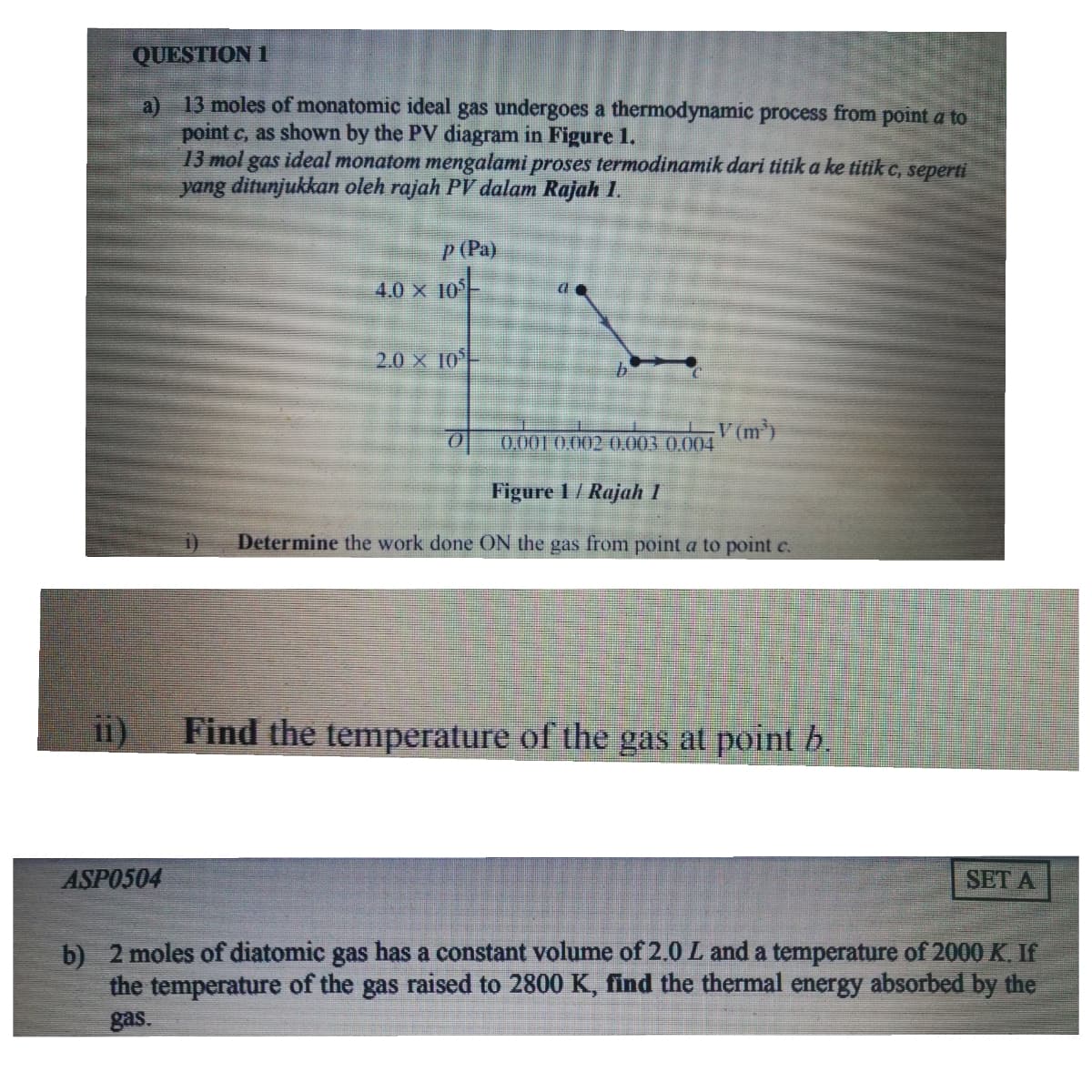
Transcribed Image Text:QUESTION 1
13 moles of monatomic ideal gas undergoes a thermodynamic process from point a to
a)
point c, as shown by the PV diagram in Figure 1.
13 mol gas ideal monatom mengalami proses termodinamik dari titik a ke titik c, seperti
yang ditunjukkan oleh rajah PV dalam Rajah 1.
P (Pa)
4.0 x 10
2.0 X 10
V (m)
0.001 0,002 0.003 0.004
Figure 1/ Rajah 1
Determine the work done ON the gas from point a to point c.
Find the temperature of the gas at point b.
i1)
ASPO504
SET A
b) 2 moles of diatomic gas has a constant volume of 2.0 L and a temperature of 2000 K. If
the temperature of the gas raised to 2800 K, find the thermal energy absorbed by the
gas.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning