Question 37 of 50 Submit Determine the Ka for an acid. To do this, construct an ICE table to determine concentrations (Part 1), and then use this information to construct and solve the equilibrium constant expression (Part 2). Complete Parts 1-2 before submitting your answer. ( PREV 1 2 Based on your ICE table (Part 1) and the definition of Ka, set up the expression for Ka. Each reaction participant must be represented by one tile. Do not combine terms. Once the expression is constructed, solve for the Ka of phenol. Ка 5 RESET [0] [0.385] [6.29] [4.09 x 10] [2.05 x 10] [5.1 x 10-7] [0.799] 1.3 x 10-10 7.8 x 10° 4.0 × 103 2.5 x 10-4 +
Question 37 of 50 Submit Determine the Ka for an acid. To do this, construct an ICE table to determine concentrations (Part 1), and then use this information to construct and solve the equilibrium constant expression (Part 2). Complete Parts 1-2 before submitting your answer. ( PREV 1 2 Based on your ICE table (Part 1) and the definition of Ka, set up the expression for Ka. Each reaction participant must be represented by one tile. Do not combine terms. Once the expression is constructed, solve for the Ka of phenol. Ка 5 RESET [0] [0.385] [6.29] [4.09 x 10] [2.05 x 10] [5.1 x 10-7] [0.799] 1.3 x 10-10 7.8 x 10° 4.0 × 103 2.5 x 10-4 +
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter16: Principles Of Chemical Reactivity: The Chemistry Of Acids And Bases
Section: Chapter Questions
Problem 127SCQ
Related questions
Question
This question is two parts and i dont understand it because on the other ones similar i put x in the ICE table but i have no x button. Can someone please help?
![Question 37 of 50
Submit
Determine the Ka for an acid.
To do this, construct an ICE table to determine concentrations (Part 1),
and then use this information to construct and solve the equilibrium
constant expression (Part 2). Complete Parts 1-2 before submitting your
answer.
( PREV
1
2
Based on your ICE table (Part 1) and the definition of Ka, set up the expression for Ka.
Each reaction participant must be represented by one tile. Do not combine terms.
Once the expression is constructed, solve for the Ka of phenol.
Ка
5 RESET
[0]
[0.385]
[6.29]
[4.09 x 10]
[2.05 x 10]
[5.1 x 10-7]
[0.799]
1.3 x 10-10
7.8 x 10°
4.0 x 103
2.5 x 10-4
+](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fb0d4a884-7750-4e06-bea4-e5f84615217d%2F6fd6ecf5-0e61-47f2-b6c5-b560474a93df%2Fklfxxps_processed.png&w=3840&q=75)
Transcribed Image Text:Question 37 of 50
Submit
Determine the Ka for an acid.
To do this, construct an ICE table to determine concentrations (Part 1),
and then use this information to construct and solve the equilibrium
constant expression (Part 2). Complete Parts 1-2 before submitting your
answer.
( PREV
1
2
Based on your ICE table (Part 1) and the definition of Ka, set up the expression for Ka.
Each reaction participant must be represented by one tile. Do not combine terms.
Once the expression is constructed, solve for the Ka of phenol.
Ка
5 RESET
[0]
[0.385]
[6.29]
[4.09 x 10]
[2.05 x 10]
[5.1 x 10-7]
[0.799]
1.3 x 10-10
7.8 x 10°
4.0 x 103
2.5 x 10-4
+
Transcribed Image Text:Question 37 of 50
Submit
Determine the Ka for an acid.
To do this, construct an ICE table to determine concentrations (Part 1),
and then use this information to construct and solve the equilibrium
constant expression (Part 2). Complete Parts 1-2 before submitting your
answer.
1
2
NEXT >
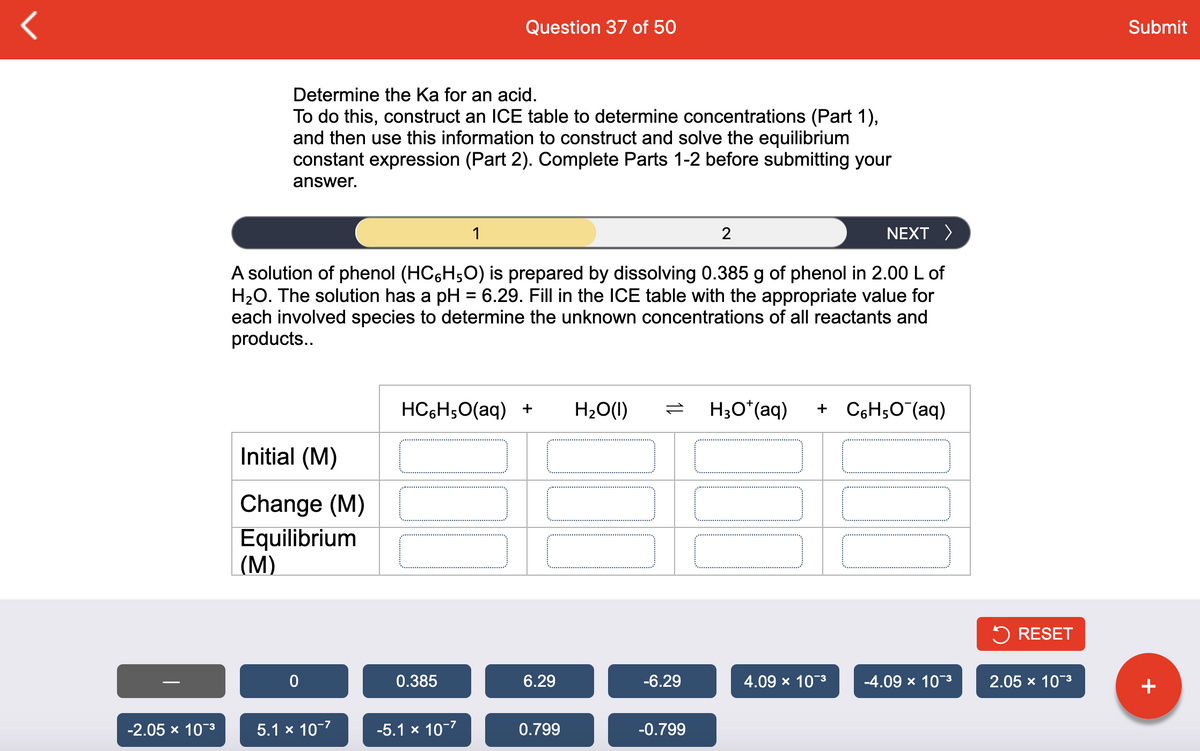
A solution of phenol (HC6H;O) is prepared by dissolving 0.385 g of phenol in 2.00 L of
H2O. The solution has a pH = 6.29. Fill in the ICE table with the appropriate value for
each involved species to determine the unknown concentrations of all reactants and
products..
HC6H;0(aq) +
H20(1)
H;O*(aq)
+ C6H5O (aq)
Initial (M)
Change (M)
Equilibrium
(М)
5 RESET
0.385
6.29
-6.29
4.09 x 10-3
-4.09 x 10-3
2.05 x 10-3
+
-2.05 х 10"3
5.1 x 10-7
-5.1 x 10-7
0.799
-0.799
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning