Safari File Edit View History Bookmarks Window Help ) 47% Sun 11:00 AM Q A session.masteringchemistry.com Consider summer class... Inbox (13) - thesym1@g... Class Schedule Listing Schedule 111.009 & 111... Ellucian Degree Works... Pearson's MyLab & Mas... ALEKS - Sofia Simmons... MasteringChemistry: H.. I Review I Constants I Periodic Table To solve stoichiometry problems, you must always calculate numbers of moles. Recall that molarity, M , is equal to the concentration in moles per liter: M = mol/L. When solutions of silver nitrate and sodium chloride are mixed, silver chloride precipitates out of solution according to the equation AGNO3 (aq) + NaCI(aq)→AgCl(s) + NaNO3 (aq) Part A What mass of silver chloride can be produced from 1.19 L of a 0.232 M solution of silver nitrate? Express your answer with the appropriate units. > View Available Hint(s) mass of AgCl = Value Units Submit Part B The reaction described in Part A required 3.31 L of sodium chloride. What is the concentration of this sodium chloride solution? Express your answer with the appropriate units. > View Available Hint(s) HÁ Value Units Submit 3141503 6. FAGE FEB átv 23 MacBook Pro
Safari File Edit View History Bookmarks Window Help ) 47% Sun 11:00 AM Q A session.masteringchemistry.com Consider summer class... Inbox (13) - thesym1@g... Class Schedule Listing Schedule 111.009 & 111... Ellucian Degree Works... Pearson's MyLab & Mas... ALEKS - Sofia Simmons... MasteringChemistry: H.. I Review I Constants I Periodic Table To solve stoichiometry problems, you must always calculate numbers of moles. Recall that molarity, M , is equal to the concentration in moles per liter: M = mol/L. When solutions of silver nitrate and sodium chloride are mixed, silver chloride precipitates out of solution according to the equation AGNO3 (aq) + NaCI(aq)→AgCl(s) + NaNO3 (aq) Part A What mass of silver chloride can be produced from 1.19 L of a 0.232 M solution of silver nitrate? Express your answer with the appropriate units. > View Available Hint(s) mass of AgCl = Value Units Submit Part B The reaction described in Part A required 3.31 L of sodium chloride. What is the concentration of this sodium chloride solution? Express your answer with the appropriate units. > View Available Hint(s) HÁ Value Units Submit 3141503 6. FAGE FEB átv 23 MacBook Pro
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
Transcribed Image Text:Safari
File Edit
View
History
Bookmarks
Window Help
) 47%
Sun 11:00 AM Q
A session.masteringchemistry.com
Consider summer class...
Inbox (13) - thesym1@g...
Class Schedule Listing
Schedule 111.009 & 111...
Ellucian Degree Works...
Pearson's MyLab & Mas...
ALEKS - Sofia Simmons...
MasteringChemistry: H..
<Homework 5
Solution Stoichiometry
18 of 24
<>
I Review I Constants I Periodic Table
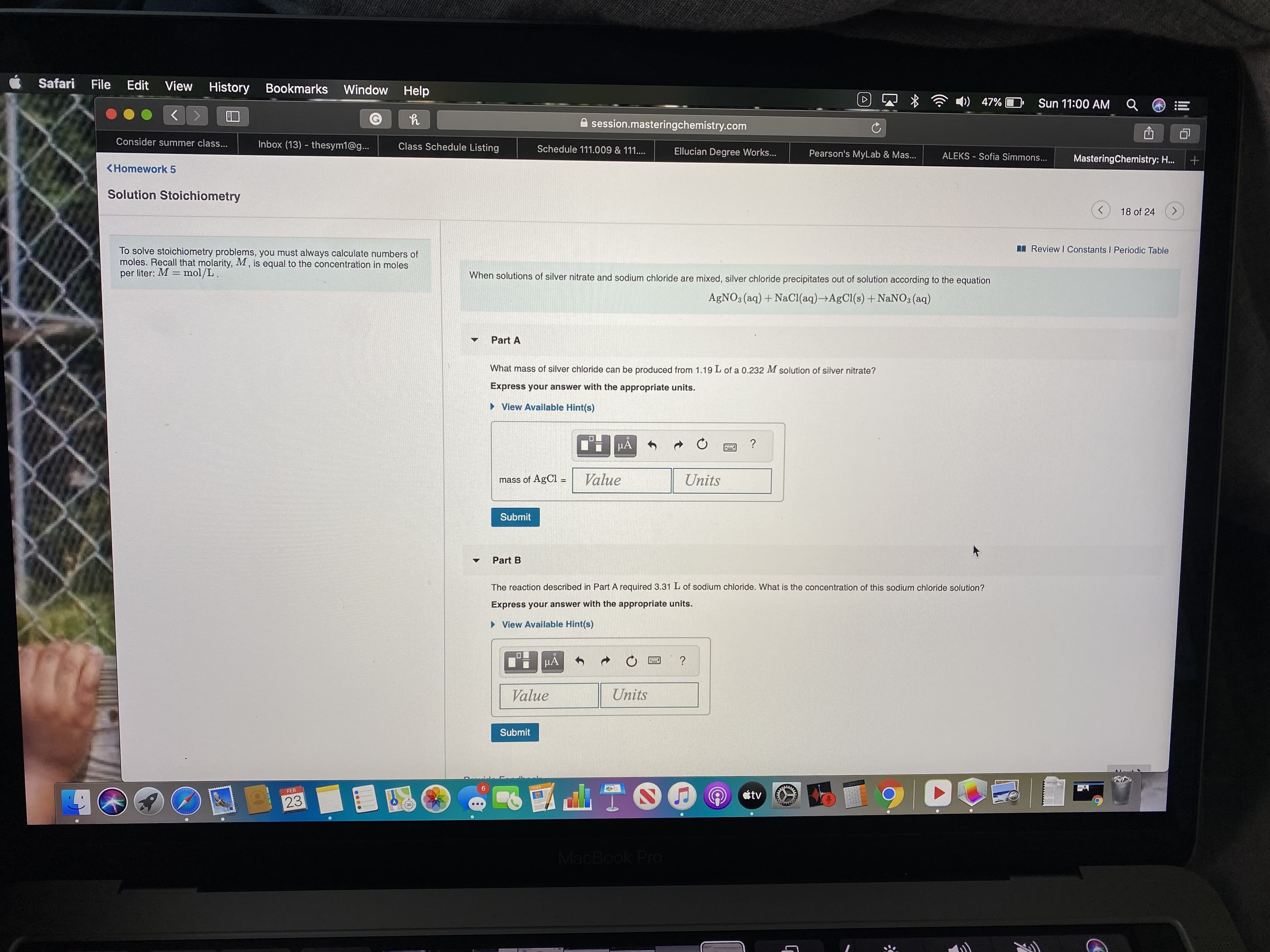
To solve stoichiometry problems, you must always calculate numbers of
moles. Recall that molarity, M , is equal to the concentration in moles
per liter: M = mol/L.
When solutions of silver nitrate and sodium chloride are mixed, silver chloride precipitates out of solution according to the equation
AGNO3 (aq) + NaCI(aq)→AgCl(s) + NaNO3 (aq)
Part A
What mass of silver chloride can be produced from 1.19 L of a 0.232 M solution of silver nitrate?
Express your answer with the appropriate units.
> View Available Hint(s)
mass of AgCl =
Value
Units
Submit
Part B
The reaction described in Part A required 3.31 L of sodium chloride. What is the concentration of this sodium chloride solution?
Express your answer with the appropriate units.
> View Available Hint(s)
HÁ
Value
Units
Submit
3141503
6.
FAGE
FEB
átv
23
MacBook Pro
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY