Separating a Mixture of Solids Are you completing this experiment online? Yes Data Collection Mass of empty beaker (g) 31.266 Mass of beaker with sample (g) 33.929 Mass of empty watch glass (g) 28.839 Mass of watch glass with iron filings(g) 29.417 Mass of clean filter paper (g) 0.464 Mass of evaporating dish (g) 35.465 Mass of evaporating dish + filter paper + sand (g) 37.306 Calculations and Analysis Total mass of mixture sample (9) 2.663 Mass and percent composition calculations Table view DList view Mass and percent composition calculations Recovered mass Iron Recovered mass Sand Calculated mass Salt 0.578 O 1.377O % Iron % Sand % Slt 21.7050 50.995O 27.3 O You did not provide a response. Percent of each component from four other students in your lab. Table view List view Percent of each component from four other students in your lab. % Iron % Sand % Salt 2
Separating a Mixture of Solids Are you completing this experiment online? Yes Data Collection Mass of empty beaker (g) 31.266 Mass of beaker with sample (g) 33.929 Mass of empty watch glass (g) 28.839 Mass of watch glass with iron filings(g) 29.417 Mass of clean filter paper (g) 0.464 Mass of evaporating dish (g) 35.465 Mass of evaporating dish + filter paper + sand (g) 37.306 Calculations and Analysis Total mass of mixture sample (9) 2.663 Mass and percent composition calculations Table view DList view Mass and percent composition calculations Recovered mass Iron Recovered mass Sand Calculated mass Salt 0.578 O 1.377O % Iron % Sand % Slt 21.7050 50.995O 27.3 O You did not provide a response. Percent of each component from four other students in your lab. Table view List view Percent of each component from four other students in your lab. % Iron % Sand % Salt 2
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter9: Liquids And Solids
Section: Chapter Questions
Problem 79QAP
Related questions
Question
Please help
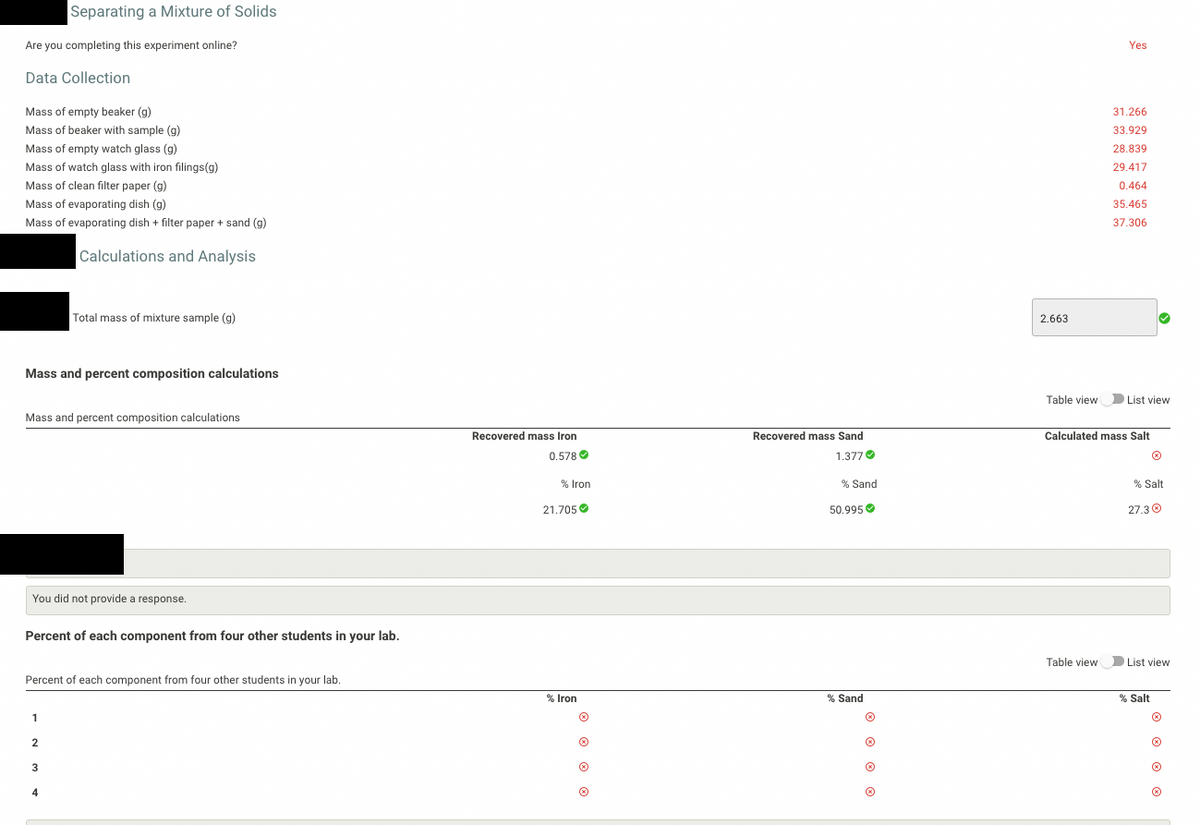
Transcribed Image Text:Separating a Mixture of Solids
Are you completing this experiment online?
Yes
Data Collection
Mass of empty beaker (g)
31.266
Mass of beaker with sample (g)
33.929
Mass of empty watch glass (g)
28.839
Mass of watch glass with iron filings(g)
29.417
Mass of clean filter paper (g)
0.464
Mass of evaporating dish (g)
35.465
Mass of evaporating dish + filter paper + sand (g)
37.306
Calculations and Analysis
Total mass of mixture sample (g)
2.663
Mass and percent composition calculations
Table view
D List view
Mass and percent composition calculations
Recovered mass Iron
Recovered mass Sand
Calculated mass Salt
0.578 O
1.377O
% Iron
% Sand
% Salt
21.705 O
50.995 O
27.30
You did not provide a response.
Percent of each component from four other students in your lab.
Table view
List view
Percent of each component from four other students in your lab.
% Iron
% Sand
% Salt
1
2
4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning