Sodium hydroxide is a better Brønsted base than sodium hydrogensulfide (NaSH), but hydrogensulfide is a better nucleophile than hydroxide. Explain in terms of definitions of basicity and nucleophilicity.
Question 1:
Sodium hydroxide is a better Brønsted base than sodium hydrogensulfide (NaSH), but hydrogensulfide is a better nucleophile than hydroxide. Explain in terms of definitions of basicity and nucleophilicity.
Question 2:
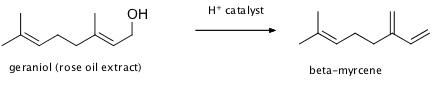
Alcohols are great starting molecules for synthesis, as well as intermediates, since they can act by turns as acids, bases, nucleophiles, or electrophiles (with proper derivatization). While fossil fuel hydrocarbons have provided us with a synthetic starting point via halogenation, Nature has provided us with the starting point of alcohols. Geraniol is an alcohol that is extractable from various plants, such as roses. While a fragrance in its own right, it is sometimes used as a feedstock to synthesize beta-myrcene, a versatile synthetic intermediate in the flavor and fragrance industry. Show a curved-arrow mechanism whereby geraniol might be transformed into beta-myrcene. It may be helpful to consider this reaction in the presence of an acid catalyst.
Trending now
This is a popular solution!
Step by step
Solved in 5 steps




