Spring 2020 rev. 10/22/2018 CHEM 51 076e Fireworks! PART B- THE FLARE! 1. Assign coefficients to balance the equation describing the burning flare: CH O(s) + KCIO,(s) _CO,(g) + H,0(g) + KCI (s) 12 22 11 precipitation, acid/base, or redox (circle one) 2. Identify the reaction type for this reaction: 3. Using the rules for assigning oxidation numbers, answer the following questions: a. Which element/atom was reduced?. .......сно к а b. Which element/atom was oxidized?. СНОК CI c. What was the reducing agent?. C12H22O11 KCIO3 CO2 H2O KCI d. What was the oxidizing agent? .. C12H22O11 KCIO3 CO2 H2O KCI e. Which element/atom gained electrons? ..C HOK CI f. How many electrons were gained by each of these atoms? g. How many electrons TOTAL were gained by these atoms in the course of one complete reaction - based on the balanced equation? .. h. Which element/atom lost electrons? . CНOKCI i. How many electrons were lost by each of these atoms?... j. How many electrons TOTAL were lost by these atoms in the course of one complete reaction - based on the balanced equation? ... k. How do the total number of electrons gained in one reaction compare to the number of electrons lost in one reaction? 4. Using the coefficients from the balanced equation, calculate the number of of potassium chlorate required to react with 4.1 g C12H22O11. Show your work. grams exothermic or endothermic 5. Given your observations, describe this reaction as: (circle one) 076-4 076e-Fireworks-Spring2020.docx Last printed 1/10/20 7:31 AM Spring 2020 rev. 10/22/2018 CHEM S1 076e Fireworks! 6. Identify the limiting reagent for this reaction in layer 1 from the reactant quantities. (HINT: How many grams of KCl could be produced from each of these reactant quantities?) 4.0 g C12H22O11 12 g KCIO3 7. Given an approximate reaction enthalpy of -9916 kJ/mol, calculate the energy change for the top-layer of the flare (in kJ). 8. A lit fuse was required to initiate the reaction in the flare. Use this information, along with your previous results to correctly label the reaction coordinate diagram shown below. Use these symbols to label each item in your diagram: reactants (R), products (P), reaction enthalpy (AH), and activation energy (Ea). Reaction Coordinate 076-5 076e-Fireworks-Spring2020.docx Last printed 1/10/20 7:31 AM
Spring 2020 rev. 10/22/2018 CHEM 51 076e Fireworks! PART B- THE FLARE! 1. Assign coefficients to balance the equation describing the burning flare: CH O(s) + KCIO,(s) _CO,(g) + H,0(g) + KCI (s) 12 22 11 precipitation, acid/base, or redox (circle one) 2. Identify the reaction type for this reaction: 3. Using the rules for assigning oxidation numbers, answer the following questions: a. Which element/atom was reduced?. .......сно к а b. Which element/atom was oxidized?. СНОК CI c. What was the reducing agent?. C12H22O11 KCIO3 CO2 H2O KCI d. What was the oxidizing agent? .. C12H22O11 KCIO3 CO2 H2O KCI e. Which element/atom gained electrons? ..C HOK CI f. How many electrons were gained by each of these atoms? g. How many electrons TOTAL were gained by these atoms in the course of one complete reaction - based on the balanced equation? .. h. Which element/atom lost electrons? . CНOKCI i. How many electrons were lost by each of these atoms?... j. How many electrons TOTAL were lost by these atoms in the course of one complete reaction - based on the balanced equation? ... k. How do the total number of electrons gained in one reaction compare to the number of electrons lost in one reaction? 4. Using the coefficients from the balanced equation, calculate the number of of potassium chlorate required to react with 4.1 g C12H22O11. Show your work. grams exothermic or endothermic 5. Given your observations, describe this reaction as: (circle one) 076-4 076e-Fireworks-Spring2020.docx Last printed 1/10/20 7:31 AM Spring 2020 rev. 10/22/2018 CHEM S1 076e Fireworks! 6. Identify the limiting reagent for this reaction in layer 1 from the reactant quantities. (HINT: How many grams of KCl could be produced from each of these reactant quantities?) 4.0 g C12H22O11 12 g KCIO3 7. Given an approximate reaction enthalpy of -9916 kJ/mol, calculate the energy change for the top-layer of the flare (in kJ). 8. A lit fuse was required to initiate the reaction in the flare. Use this information, along with your previous results to correctly label the reaction coordinate diagram shown below. Use these symbols to label each item in your diagram: reactants (R), products (P), reaction enthalpy (AH), and activation energy (Ea). Reaction Coordinate 076-5 076e-Fireworks-Spring2020.docx Last printed 1/10/20 7:31 AM
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter20: Environmental Chemistry-earth's Environment, Energy, And Sustainability
Section: Chapter Questions
Problem 41PS
Related questions
Question

Transcribed Image Text:Spring 2020
rev. 10/22/2018
CHEM 51
076e Fireworks!
PART B- THE FLARE!
1. Assign coefficients to balance the equation describing the burning flare:
CH O(s) +
KCIO,(s)
_CO,(g) +
H,0(g) +
KCI (s)
12
22 11
precipitation, acid/base, or redox
(circle one)
2. Identify the reaction type for this reaction:
3. Using the rules for assigning oxidation numbers, answer the following questions:
a. Which element/atom was reduced?.
.......сно к а
b. Which element/atom was oxidized?.
СНОК CI
c. What was the reducing agent?.
C12H22O11 KCIO3 CO2 H2O KCI
d. What was the oxidizing agent? ..
C12H22O11 KCIO3 CO2 H2O KCI
e. Which element/atom gained electrons?
..C HOK CI
f. How many electrons were gained by each of these atoms?
g. How many electrons TOTAL were gained by these atoms in the course of one
complete reaction - based on the balanced equation? ..
h. Which element/atom lost electrons?
. CНOKCI
i. How many electrons were lost by each of these atoms?...
j. How many electrons TOTAL were lost by these atoms in the course of one
complete reaction - based on the balanced equation? ...
k. How do the total number of electrons gained in one reaction compare to the
number of electrons lost in one reaction?
4. Using the coefficients from the balanced equation, calculate the number of
of potassium chlorate required to react with 4.1 g C12H22O11. Show your work.
grams
exothermic or endothermic
5. Given your observations, describe this reaction as:
(circle one)
076-4
076e-Fireworks-Spring2020.docx
Last printed 1/10/20 7:31 AM
Transcribed Image Text:Spring 2020
rev. 10/22/2018
CHEM S1
076e Fireworks!
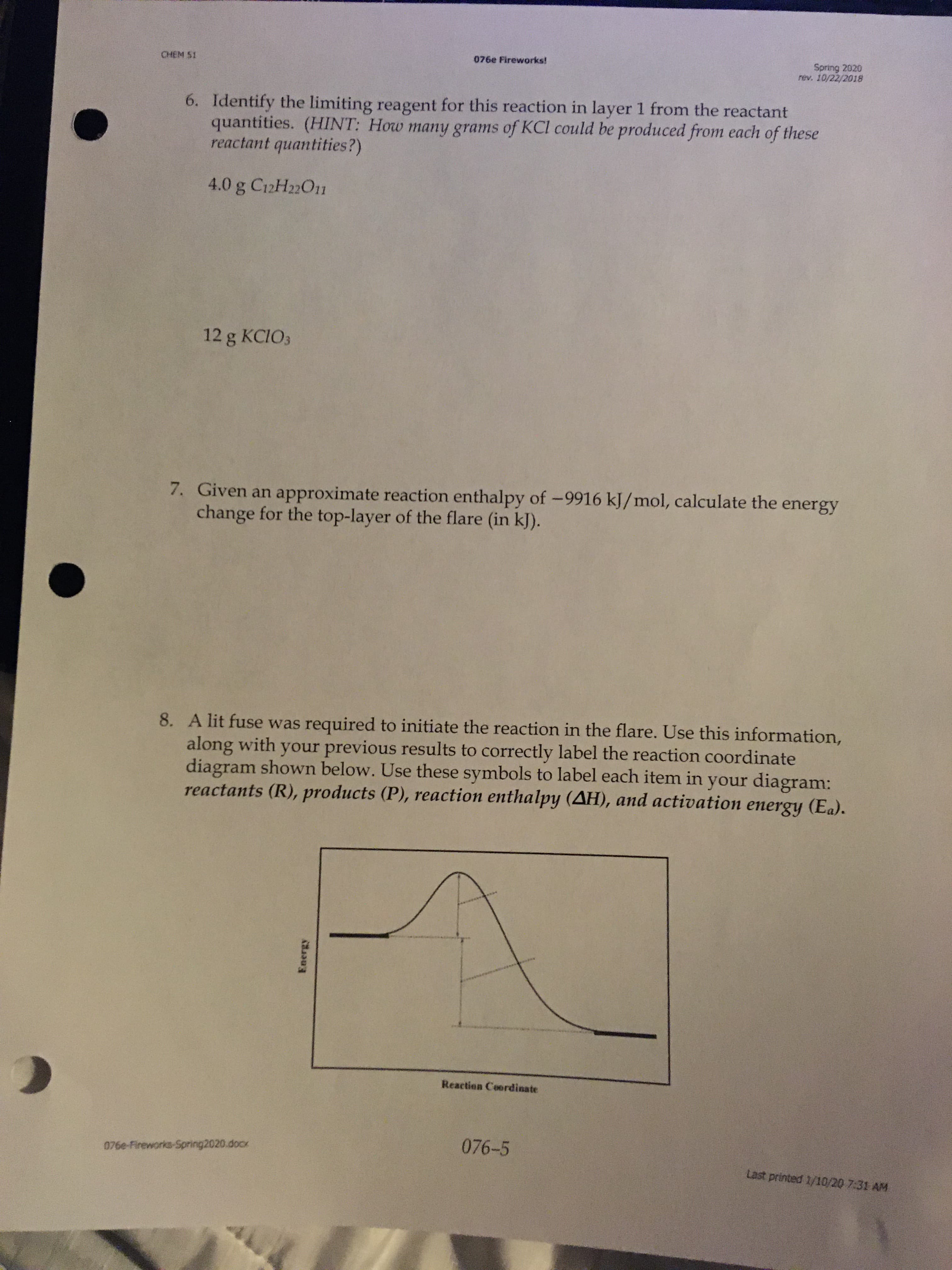
6. Identify the limiting reagent for this reaction in layer 1 from the reactant
quantities. (HINT: How many grams of KCl could be produced from each of these
reactant quantities?)
4.0 g C12H22O11
12 g KCIO3
7. Given an approximate reaction enthalpy of -9916 kJ/mol, calculate the energy
change for the top-layer of the flare (in kJ).
8. A lit fuse was required to initiate the reaction in the flare. Use this information,
along with your previous results to correctly label the reaction coordinate
diagram shown below. Use these symbols to label each item in your diagram:
reactants (R), products (P), reaction enthalpy (AH), and activation energy (Ea).
Reaction Coordinate
076-5
076e-Fireworks-Spring2020.docx
Last printed 1/10/20 7:31 AM
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning