Standardization of Hydrochloric Acid Solution Answer the concentrations in 3 significant figure. The tolerance or error should follow the decimal place of the value. You can use scientific notation using "e". Example: 0.0010 = 1.0e-3 Primary Standard: Na2C03 Formula Mass of 1° Standard: 105.99 g/mol Purity of 1° Standard: 95% Instrument used: Analytical Balance Trial 1 3 0.0504 + 1° Standard 0.0510 (same 0.0501 (same weight, g as 1) as 1) Net vol. of 18.00 ± 0.14 18.30 ± 0.14 17.90 ± 0.14 HCI, mL M HCI Average M HCI
Standardization of Hydrochloric Acid Solution Answer the concentrations in 3 significant figure. The tolerance or error should follow the decimal place of the value. You can use scientific notation using "e". Example: 0.0010 = 1.0e-3 Primary Standard: Na2C03 Formula Mass of 1° Standard: 105.99 g/mol Purity of 1° Standard: 95% Instrument used: Analytical Balance Trial 1 3 0.0504 + 1° Standard 0.0510 (same 0.0501 (same weight, g as 1) as 1) Net vol. of 18.00 ± 0.14 18.30 ± 0.14 17.90 ± 0.14 HCI, mL M HCI Average M HCI
Chapter12: Water Requirements For Aquaculture
Section: Chapter Questions
Problem 27SA
Related questions
Question
Given the following data, what is the molarity? Include the relative error
Error is 1.0 mg or 1.0e-4
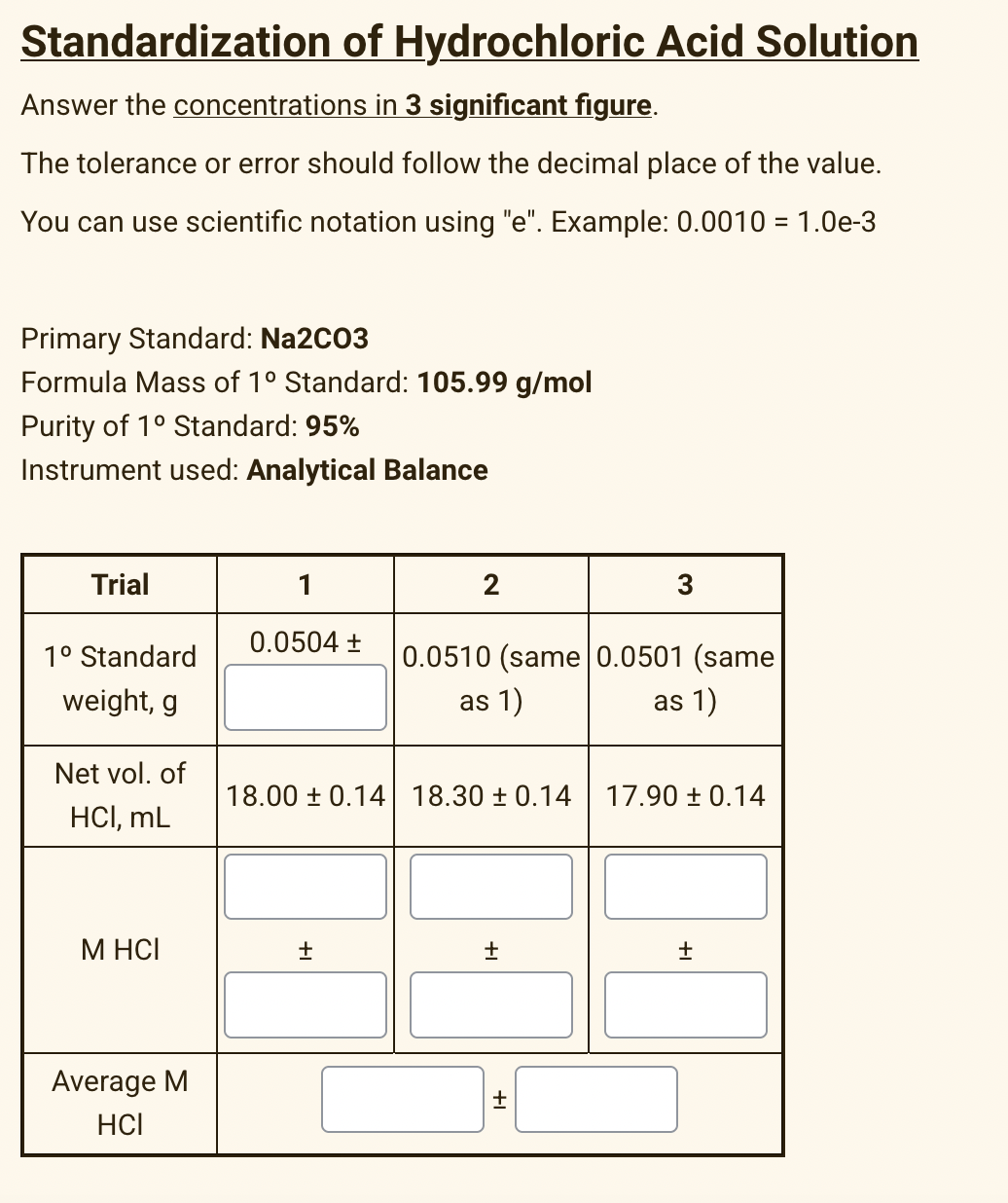
Transcribed Image Text:Standardization of Hydrochloric Acid Solution
Answer the concentrations in 3 significant figure.
The tolerance or error should follow the decimal place of the value.
You can use scientific notation using "e". Example: 0.0010 = 1.0e-3
Primary Standard: Na2C03
Formula Mass of 1° Standard: 105.99 g/mol
Purity of 1° Standard: 95%
Instrument used: Analytical Balance
Trial
1
2
3
0.0504 +
1° Standard
0.0510 (same 0.0501 (same
weight, g
as 1)
as 1)
Net vol. of
18.00 ± 0.14 18.30 ± 0.14
17.90 ± 0.14
HCI, mL
M HCI
Average M
HCI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Recommended textbooks for you



