Strontium phosphate, Sr3(PO.)2 is a crystalline substance used in medicine and industry. How many Phosphorus atoms are represented in the formulas for Sr3(PO4)2? 0/ 10000 Word Limit II !!!
Strontium phosphate, Sr3(PO.)2 is a crystalline substance used in medicine and industry. How many Phosphorus atoms are represented in the formulas for Sr3(PO4)2? 0/ 10000 Word Limit II !!!
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter3: Molecules, Moles, And Chemical Equations
Section: Chapter Questions
Problem 3.69PAE
Related questions
Question
100%
Please help.
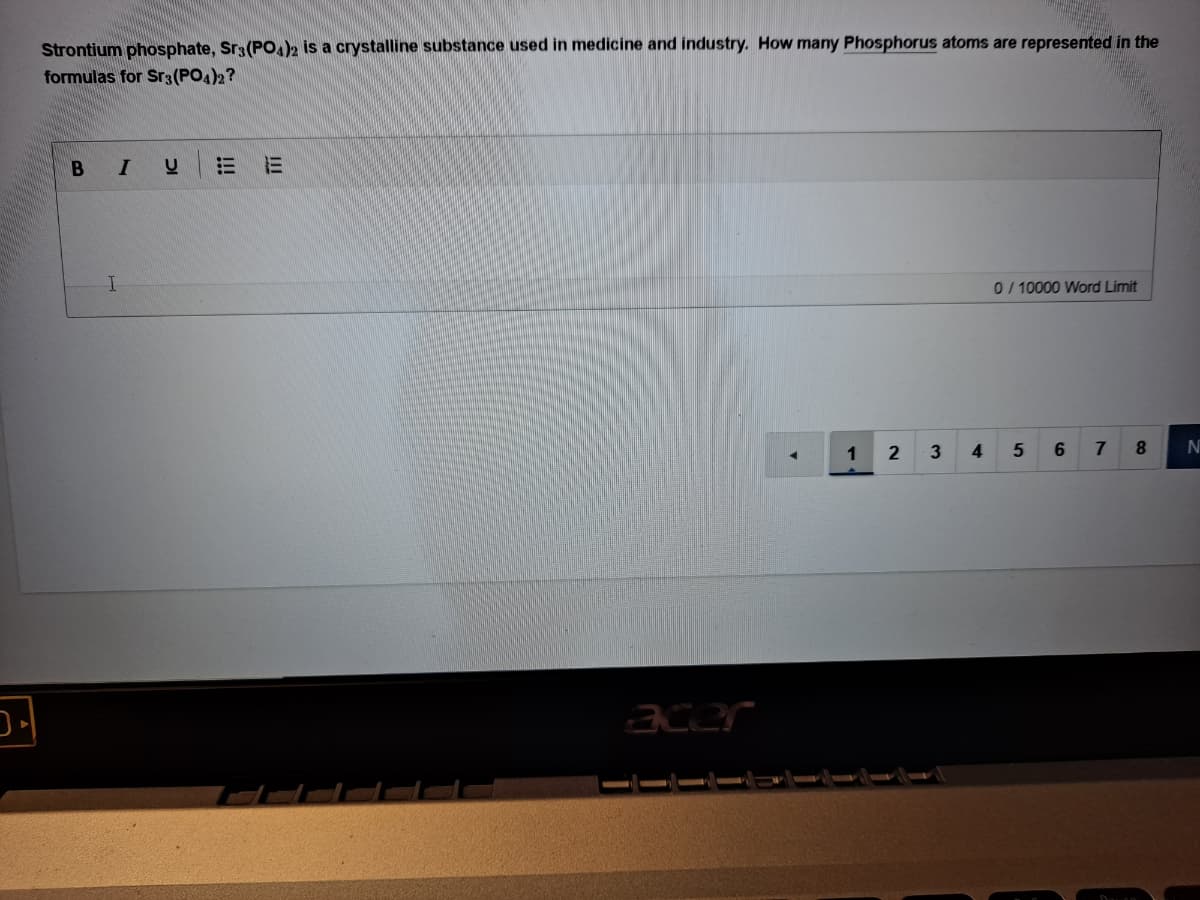
Transcribed Image Text:Strontium phosphate, Sr3(PO.)2 is a crystalline substance used in medicine and industry. How many Phosphorus atoms are represented in the
formulas for Sr3(PO4)2?
三
0/ 10000 Word Limit
1
2
3
4
8.
N
ן-כ
!!!
Expert Solution
Step 1
The given compound is Sr3(PO4)2.
We have calculate number of P atoms present in that molecule.
The subscript following each atom (or atom cluster, called a polyatomic ion)indicates the number of atoms preceding it in one molecule of the substance.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning