Table 15.3 Maximum-boiling-point azeotropes Azeotrope Composition (Weight Percentage) Boiling Point (°C) 20.0% CH,COCH,, 80.0% CHCI, 17.0% CHCI, 83.0% CH,COCH,CH, 20.2% HCI, 79.8% H,О 77.0% CH,COCH, 23.0% C̟H¿O, 49.0% C,H,СНО, 51.0% CH,ОН Acetone-chloroform 64.7 Chloroform-methyl ethyl ketone 79.9 Hydrochloric acid 108.6 Acetic acid-dioxane 119.5 Benzaldehyde-phenol 185.6
Table 15.3 Maximum-boiling-point azeotropes Azeotrope Composition (Weight Percentage) Boiling Point (°C) 20.0% CH,COCH,, 80.0% CHCI, 17.0% CHCI, 83.0% CH,COCH,CH, 20.2% HCI, 79.8% H,О 77.0% CH,COCH, 23.0% C̟H¿O, 49.0% C,H,СНО, 51.0% CH,ОН Acetone-chloroform 64.7 Chloroform-methyl ethyl ketone 79.9 Hydrochloric acid 108.6 Acetic acid-dioxane 119.5 Benzaldehyde-phenol 185.6
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter7: Equilibria In Multiple-component Systems
Section: Chapter Questions
Problem 7.35E
Related questions
Question
Construct an approximate boiling-point-composition diagram for a benzene– methanol system. The mixture shows azeotropic behavior (see Table 15.3). Include on the graph the boiling points of pure benzene and pure methanol and the boiling point of the azeotrope. Describe the behavior on distillation of a mixture that is initially rich in benzene (90%) and then for a mixture that is initially rich in methanol (90%).
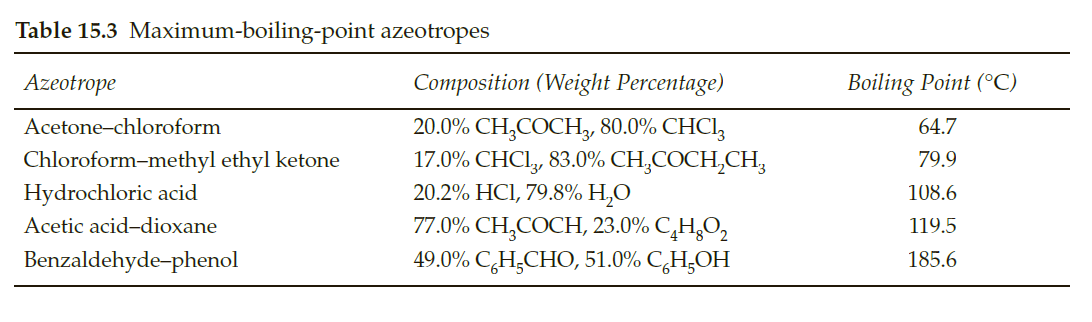
Transcribed Image Text:Table 15.3 Maximum-boiling-point azeotropes
Azeotrope
Composition (Weight Percentage)
Boiling Point (°C)
20.0% CH,COCH,, 80.0% CHCI,
17.0% CHCI, 83.0% CH,COCH,CH,
20.2% HCI, 79.8% H,О
77.0% CH,COCH, 23.0% C̟H¿O,
49.0% C,H,СНО, 51.0% CH,ОН
Acetone-chloroform
64.7
Chloroform-methyl ethyl ketone
79.9
Hydrochloric acid
108.6
Acetic acid-dioxane
119.5
Benzaldehyde-phenol
185.6
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,