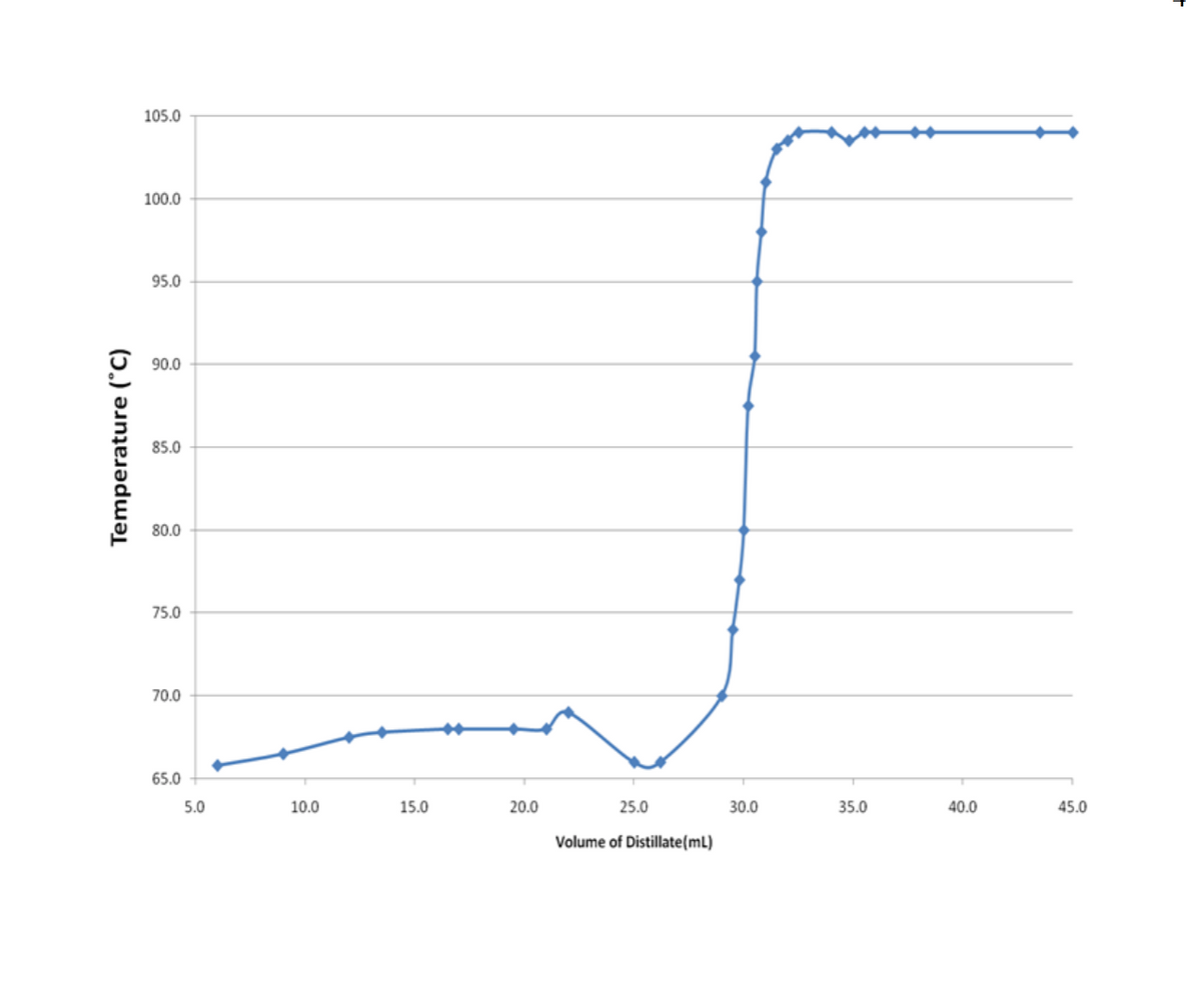
7.A student carried out a fractional distillation of an unknown binary (2-component) mixture from a 50 mL pot solution. Based on the plot below, and assuming no azeotropes, what percent by volume was the solution in the lower boiling point component?
7.A student carried out a fractional distillation of an unknown binary (2-component) mixture from a 50 mL pot solution. Based on the plot below, and assuming no azeotropes, what percent by volume was the solution in the lower boiling point component?
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter11: Solutions And Colloids
Section: Chapter Questions
Problem 27E: What is the microscopic explanation for the macroscopic behaviour illustrated in Figure 11.15?
Related questions
Question
7.A student carried out a fractional distillation of an unknown binary (2-component) mixture from a 50 mL pot solution. Based on the plot below, and assuming no azeotropes, what percent by volume was the solution in the lower boiling point component?
Transcribed Image Text:105.0
100.0
95.0
90.0
85.0
80.0
75.0
70.0
65.0
5.0
10.0
15.0
20.0
25.0
30.0
35.0
40.0
45.0
Volume of Distillate(mL)
Temperature (°C)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning