Table 9.2 Energy Length Bond Single Bonds H-H H-F H-Cl H-Br H-I C-H C-C C-Si C-N C-0 432 565 427 363 295 413 347 301 305 358 264 259 453 339 C-P C-S C-F C-Cl C-Br 276 C-I 216 Multiple Bonds C=C 614 C=N 615 C=0 745 (799 in CO₂) 74 92 127 141 161 109 154 186 147 143 187 181 133 177 194 213 134 127 123 Bond Energy Length N-H N-N N-P N-O N-F N-Cl N-Br N-I O-H O-P 0-0 O-S O-F O-Cl O-Br 0-I N=N N=O 0₂ 391 160 209 201 272 200 243 159 467 351 204 265 190 203 234 234 418 607 498 101 146 177 144 139 191 214 222 96 160 148 151 142 164 172 194 122 120 121 Bond Si-H Si-Si Si-O Si-S Si-F Si-Cl Si-Br Si-I P-H P-Si P-P P-F P-Cl P-Br P-I Energy Length C=C C=N C=O 323 226 368 226 565 381 310 234 320 213 200 490 331 272 184 839 891 1070 148 234 161 210 156 204 216 240 142 227 221 156 204 222 246 121 115 113 Bond S-H S-S S-F S-CI S-Br S-I F-F F-Cl F-Br F-I Energy Length Cl-Cl Cl-Br Cl-I Br-Br Br-I 1-1 N=N ΝΞΟ 347 266 327 271 218 ~170 159 193 212 263 243 215 208 193 175 151 945 1020 134 204 158 201 225 234 143 166 178 187 199 214 243 228 248 266 110 106
Formal Charges
Formal charges have an important role in organic chemistry since this concept helps us to know whether an atom in a molecule is neutral/bears a positive or negative charge. Even if some molecules are neutral, the atoms within that molecule need not be neutral atoms.
Polarity Of Water
In simple chemical terms, polarity refers to the separation of charges in a chemical species leading into formation of two polar ends which are positively charged end and negatively charged end. Polarity in any molecule occurs due to the differences in the electronegativities of the bonded atoms. Water, as we all know has two hydrogen atoms bonded to an oxygen atom. As oxygen is more electronegative than hydrogen thus, there exists polarity in the bonds which is why water is known as a polar solvent.
Valence Bond Theory Vbt
Valence bond theory (VBT) in simple terms explains how individual atomic orbitals with an unpaired electron each, come close to each other and overlap to form a molecular orbital giving a covalent bond. It gives a quantum mechanical approach to the formation of covalent bonds with the help of wavefunctions using attractive and repulsive energies when two atoms are brought from infinity to their internuclear distance.
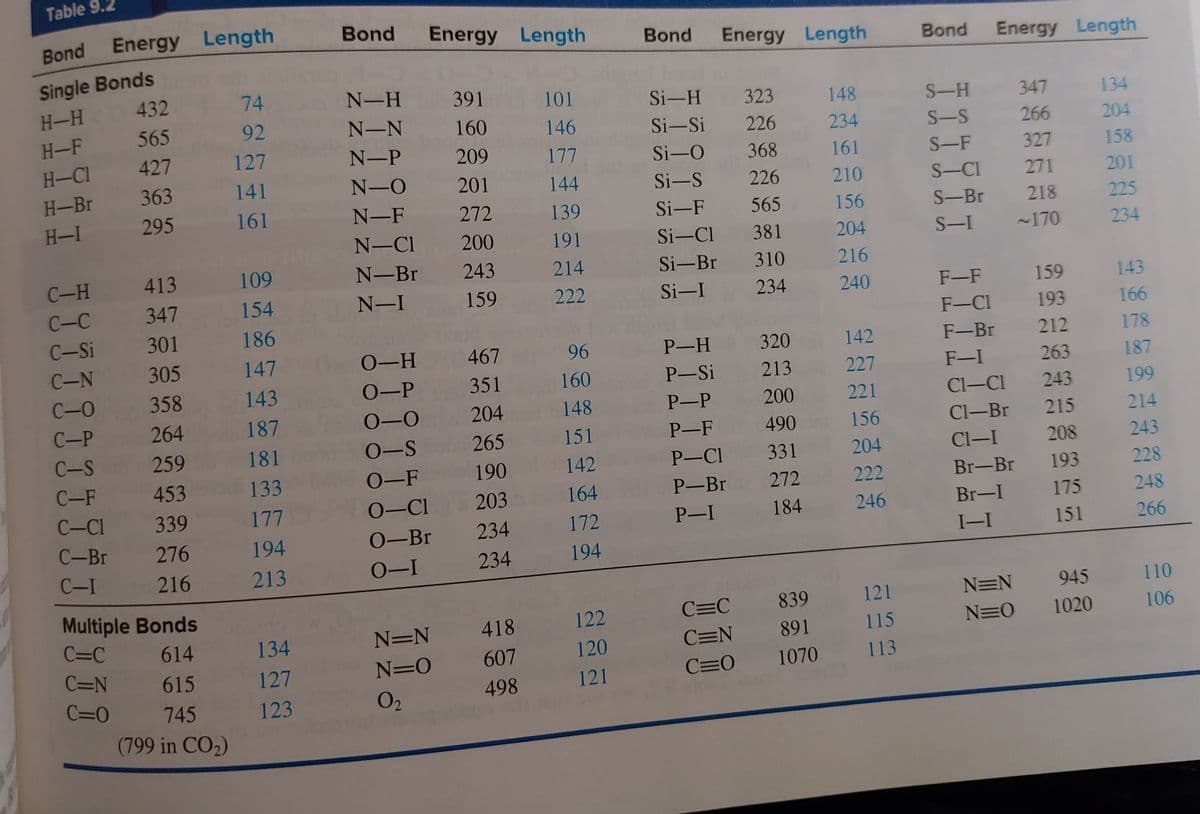
Use a bond energy table to determine the amount of heat energy released per mole of propane
when it reacts with oxygen to form carbon dioxide and water. (You will need to balance the
reaction and look up a bond energy table) Show your work.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images









